Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's disease: Clinical trials, pharmacology and treatment
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To characterize the peripheral pharmacokinetics and CNS metabolism of levodopa during continuous subcutaneous delivery of solubilized carbidopa.
Background: Although many commercial levodopa formulations include carbidopa for gastrointestinal dopa decarboxylase inhibition, little is known how manipulating carbidopa delivery affects the pharmacokinetics of levodopa.
Methods: We conducted a series of pharmacokinetic studies in pigs, mice, and humans to characterize effects of continuous subcutaneous carbidopa delivery co-administered with oral levodopa/carbidopa (IR-LD/CD) compared to oral IR-LD/CD on levodopa pharmacokinetics. The porcine and human studies compared peripheral levodopa pharmacokinetic parameters and the mouse studies compared brain levodopa and dopamine levels.
Results: In pigs receiving oral IR-LD/CD (125/25mg, TID), additional continuous subcutaneous carbidopa delivery (60mg/24h) significantly increased the levodopa t1/2 and AUC versus IR-LD/CD alone, and versus IR-LD/CD plus oral carbidopa at doses equivalent to those administered subcutaneously. In mice, continuous administration of carbidopa (0.5mg/24h) in addition to oral IR-LD/CD (1.2/0.3 mg BID) improved peripheral levodopa pharmacokinetics as well as brain dopamine concentrations, with no significant effect on brain levodopa levels. We also confirmed that carbidopa given at relatively high and constant rates of delivery does not inhibit levodopa decarboxylation in the brain. In healthy human volunteers receiving oral IR-LD/CD (200/50mg BID), subcutaneous continuous administration of carbidopa increased peripheral levodopa bioavailability (Table).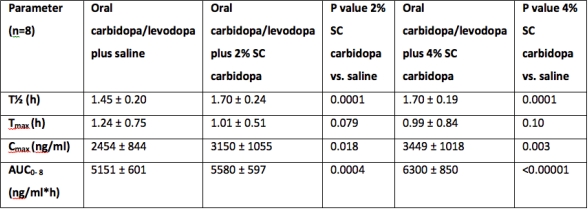
Conclusions: This series of studies demonstrates that maintaining basal plasma concentrations of carbidopa are essential for attaining maximum plasma concentrations of levodopa. Taken together, the studies indicate that parenterally administered carbidopa leads to greater inhibition than achieved after even larger doses of orally- administered carbidopa.
To cite this abstract in AMA style:
R. Shaltiel-Karyo, E. Zawoznik, I. Weinstock, M. Nemas, Y. Caraco, P.A. LeWitt, S. Oren, O. Yacoby-Zeevi. Continuous subcutaneous delivery of solubilized carbidopa improves the pharmacokinetic profile of levodopa [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/continuous-subcutaneous-delivery-of-solubilized-carbidopa-improves-the-pharmacokinetic-profile-of-levodopa/. Accessed January 16, 2026.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/continuous-subcutaneous-delivery-of-solubilized-carbidopa-improves-the-pharmacokinetic-profile-of-levodopa/
