Category: Tremor
Objective: To report preliminary results on the safety and efficacy of staged bilateral magnetic resonance-guided focused ultrasound (MRgFUS) thalamotomy for the treatment of essential tremor (ET).
Background: Focused ultrasound unilateral thalamotomy is an approved treatment for ET and has shown to improve both disability and quality of life1. One unanswered question is whether MRgFUS bilateral thalamic ablation can be tolerated, as bilateral radiofrequency thalamotomy was abandoned in the past due to a high rate of worsening of speech and balance2. However, the possibility of performing bilateral lesions with a less invasive technique such as focused ultrasound deserves to reconsider this approach.
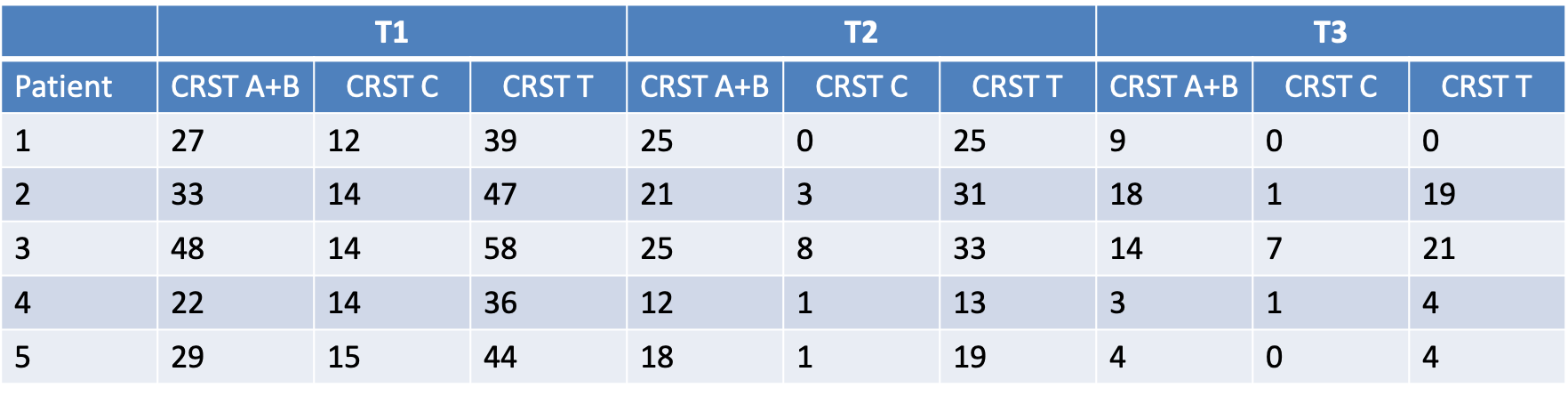
Method: 5 patients who had received unilateral MRgFUS thalamotomy at least one year before and who had tremor in the non-treated hand underwent a second thalamotomy on the contralateral hemisphere. The first thalamotomy had not resulted in any permanent side effect and had achieved tremor control in the treated hand. Safety of the second lesion was based on an evaluation of observed and/or reported treatment-related side effects. Additionally, complete neuropsychological testing and voice and gait kinematic analysis were performed. The benefit of the second treatment was assessed through the CRST which includes part A (examination of tremor in each body part), B (task performance) and C (independence for activities of daily living). Three main timepoints were considered: baseline before first thalamotomy (T1); baseline before second thalamotomy (T2); and 6 months after second thalamotomy (T3). Presentation of the data is purely descriptive.
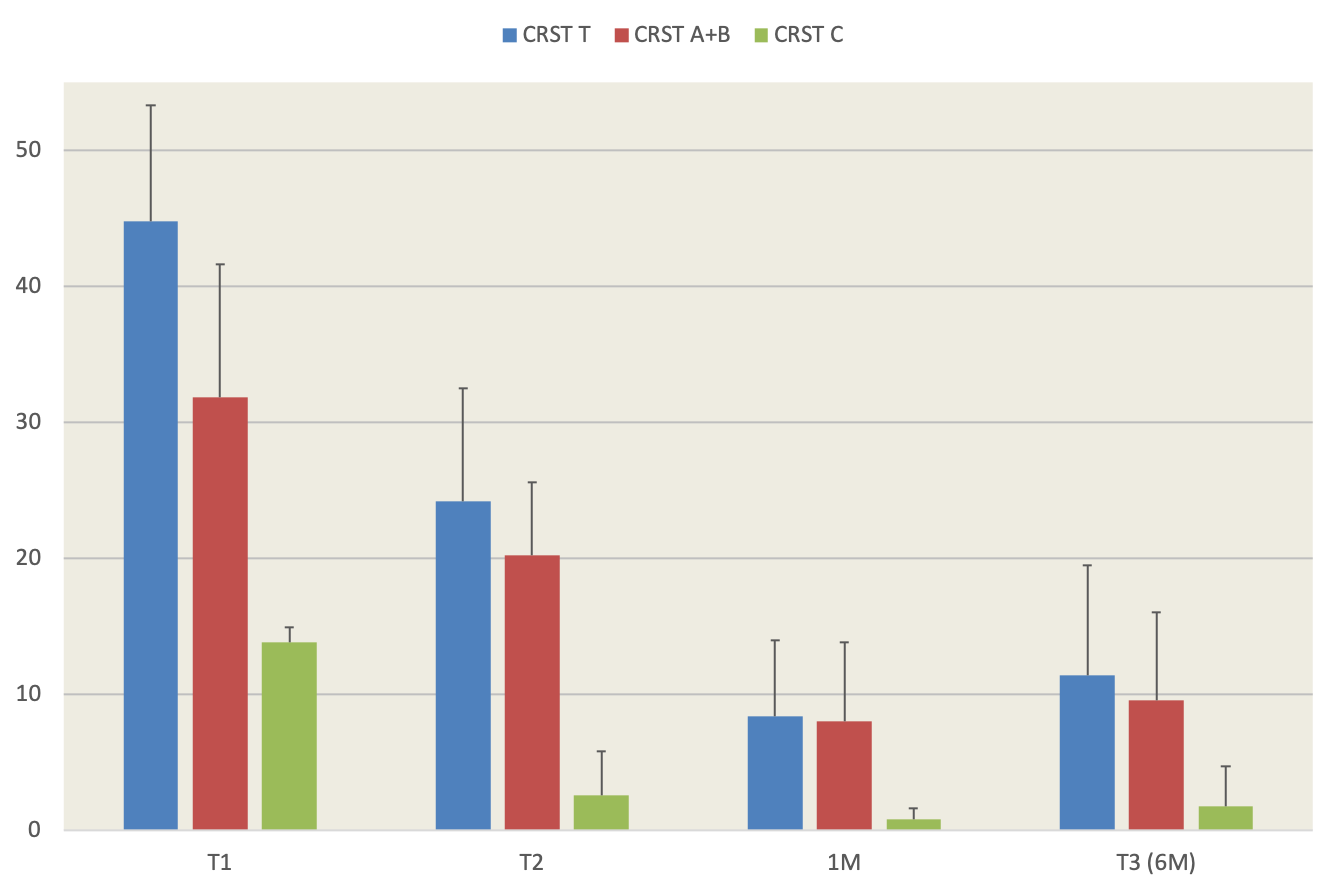
Results: No permanent side effects were registered. All five patients reported mild and transient gait instability after second treatment which resolved within the first few weeks in all cases. One patient reported dysgeusia for a few days following bilateral thalamic ablation. Neither neuropsychological assessment nor voice and kinematic analysis detected any worsening after second thalamotomy as compared to T2. CRST A+B improved by 71% from T1 to T3 (31,8±9,8 to 6,4±9,6) while part C decreased by 87% (13,8±1,0 to 1,8±2,9) [figure 1]. Improvements from T2 to T3 were 55% for parts A+B and 35% for CRST C.
Conclusion: This is the first evidence that MRgFUS bilateral staged thalamotomy is safe and can be applied to treat ET. A larger trial is warranted to confirm this preliminary data.
References: 1. Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016. doi:10.1056/NEJMoa1600159 2. Alshaikh J, Fishman PS. Revisiting bilateral thalamotomy for tremor. Clin Neurol Neurosurg. 2017. doi:10.1016/j.clineuro.2017.04.025
To cite this abstract in AMA style:
R. Martinez-Fernandez, J. Pineda-Pardo, J. Mañez-Miró, M. del Álamo, F. Hernandez-Fernandez, R. Rodriguez-Rojas, L. Guida, M. Matarazzo, L. Hermes-Gonzalez, L. Diaz-Fernandez, C. Ammann, L. Vela, J. Obeso. Safety and efficacy of focused ultrasound staged bilateral thalamotomy for Essential tremor [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-focused-ultrasound-staged-bilateral-thalamotomy-for-essential-tremor/. Accessed December 24, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-focused-ultrasound-staged-bilateral-thalamotomy-for-essential-tremor/