Objective: To evaluate a reduction of LED in PD patients who achieve satisfactory motor control with long-term continuous subcutaneous apomorphine infusion (CSAI)
Background: Apomorphine is a potent dopamine agonist useful in the treatment of Parkinson’s disease (PD) patients with disabling motor fluctuations with frequent ‘off’ periods. By providing a more continuous dopaminergic delivery, apomorphine potentially offers the capacity to lower levodopa equivalence dosage (LED) whilst achieving motor symptoms control.
Method: Here, we report data from the Thai Apomorphine Registry for the first 50 patients undergoing CSAI therapy (www.aporegistry.org). The multidisciplinary teams involved in patients’ care collected and uploaded data to the registry to enable treatment monitoring and analysis using electronic datasheets. All recorded data was collected for further analysis.
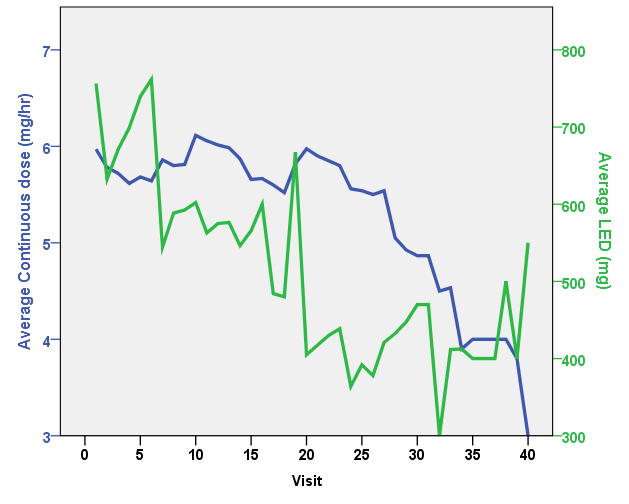
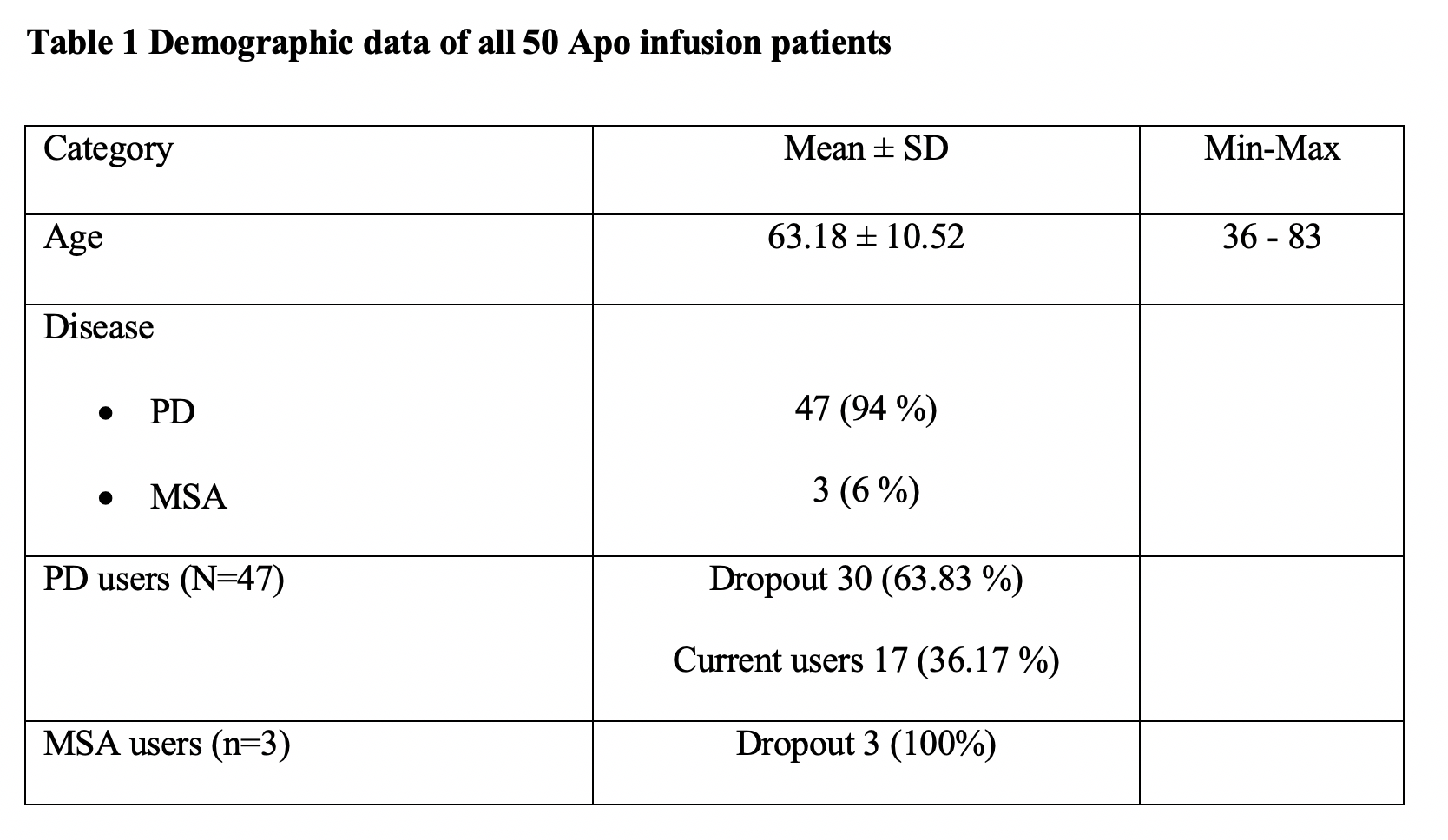
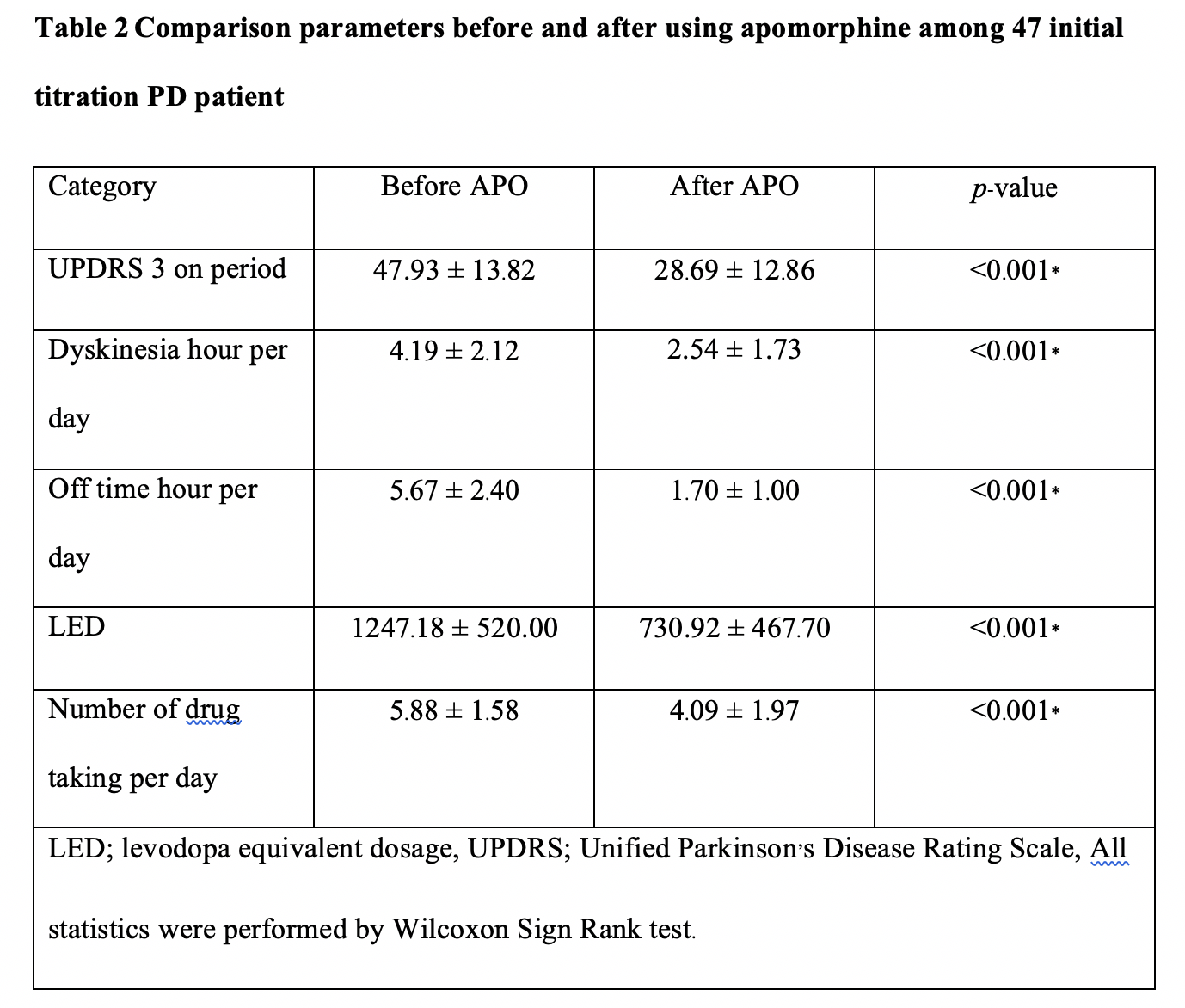
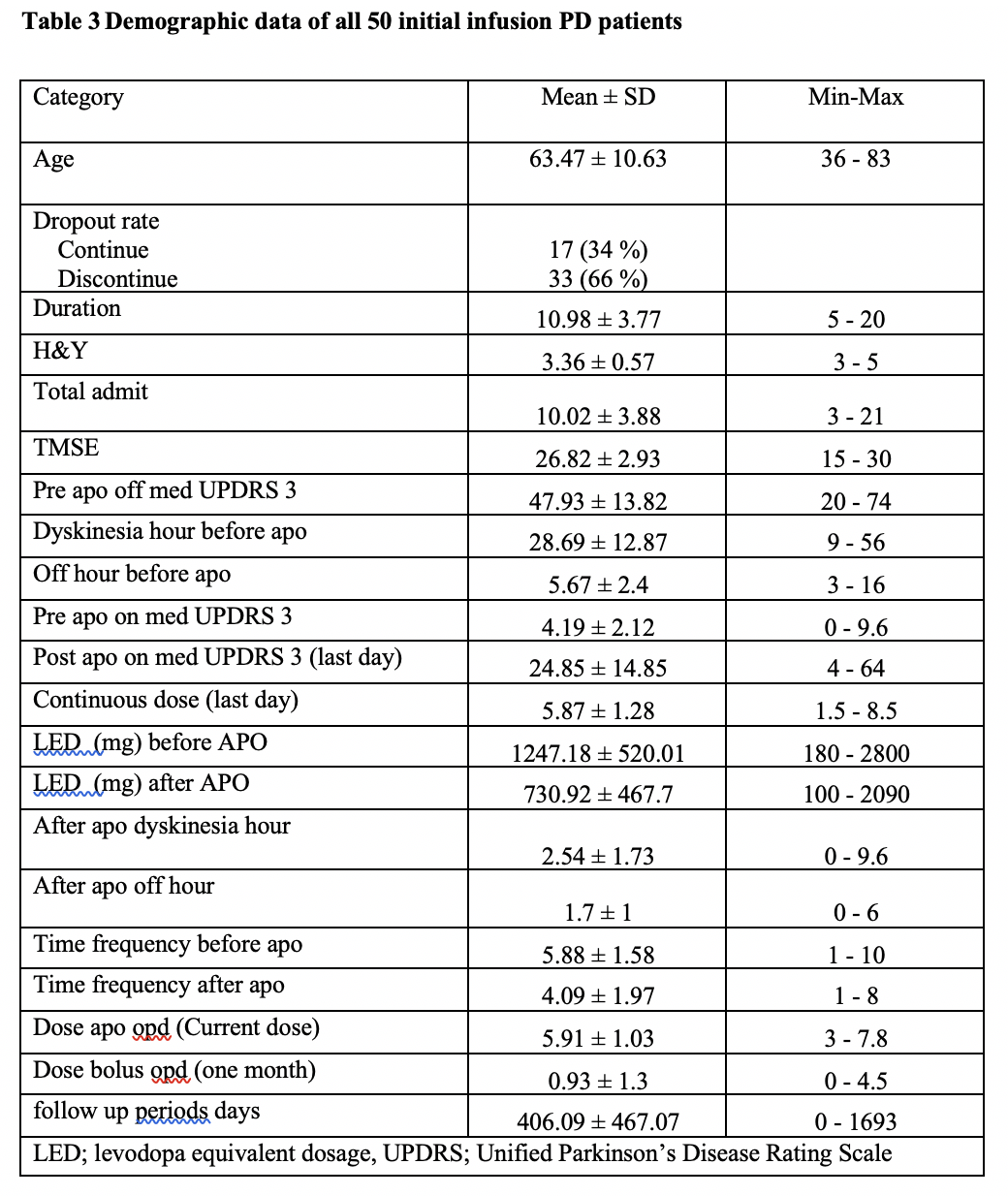
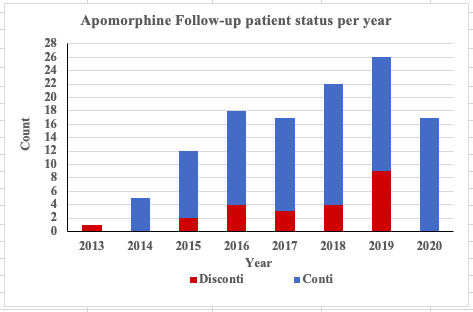
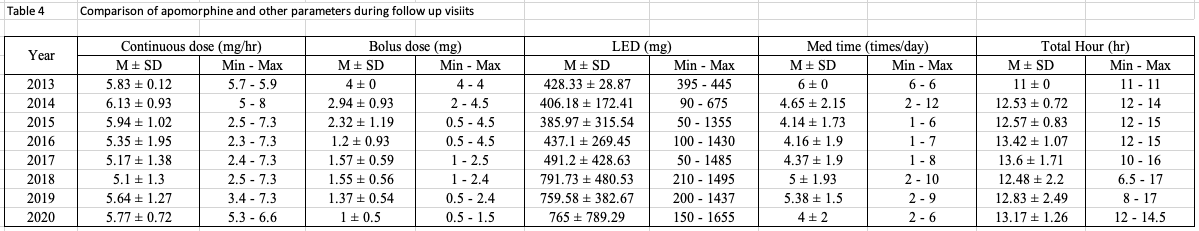
Results: A total of 47 PD patients have undergone CSAI treatment. Following initiation, PD patients were discharged on a mean (± SD) dose of 5.87 ± 1.28 mg/hour. The mean (± SD) follow-up period was 406.09 ± 467.07 days. All patients showed significant improvement in the UPDRS score, daily OFF time and dyskinesia, as well as reductions in LED and the daily intake of oral PD medication versus pre-treatment values (p<0.05). 66% of PD patients discontinued therapy during the follow-up period. Seventeen current CSAI users reported continuous benefits of CSAI overtime, with a trend towards substantial decreases in the continuous dosage of CSAI without a significant increment of oral dopaminergic medication whilst maintaining good motoric outcomes. This finding supports the hypothesis that of providing continuous dopaminergic delivery with apomorphine can potentially widen the therapeutic window in PD, as well as provide the capability to lower LED. This concept provides a rationale for considering early infusion amongst PD patients who experience significant motor fluctuations.
Conclusion: Long-term CSAI therapy provides immediate and long-term benefits that offer the opportunity for patients to lower their total dopaminergic medications over time. Using Thai Apomorphine Registry allow standardised, prospective collection of patient data and continuous follow-up from the time of initiation and affording a valuable resource for future research.
References: Katzenschlager R, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2018; 17( 9): 749-759. Antonini A, et al. Pros and Cons of Apomorphine and L-dopa continuous infusion in advanced Parkinson’s disease. Parkinsonism and Related Disorders 2009;15(4):S97-S100.
To cite this abstract in AMA style:
O. Phokaewvarangkul, K. Boonpang, R. Bhidayasiri. Apomorphine subcutaneous infusion reduces dopaminergic dosage whilst maintaining motor benefits in Parkinson’s disease: A prospective analysis of Thai Apomorphine Registry [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/apomorphine-subcutaneous-infusion-reduces-dopaminergic-dosage-whilst-maintaining-motor-benefits-in-parkinsons-disease-a-prospective-analysis-of-thai-apomorphine-registry/. Accessed April 4, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/apomorphine-subcutaneous-infusion-reduces-dopaminergic-dosage-whilst-maintaining-motor-benefits-in-parkinsons-disease-a-prospective-analysis-of-thai-apomorphine-registry/