Objective: To determine whether new chemical modifications of the previously described allosteric GCase chaperone N607 (Aflaki E et.al Neurosc. 2016) increase GCase activity in monocyte-derived macrophages from GBA-PD patients.
Background: Mutations in the glucocerebrosidase gene (GBA) are the most common cause of Parkinson’s disease (PD). GBA encodes the lysosomal enzyme glucocerebrosidase (GCase). There is no therapy for PD associated with mutations in the GBA gene (GBA-PD). Last data showed that small molecule chaperones as ambroxol or isofagomine (IFG) could cross the BBB and help mutant GCase refold and traffic correctly to lysosomes. However, both compounds bind to GCase active site. Allosteric GCase molecular chaperones could be more effective in restoring of GCase activity.
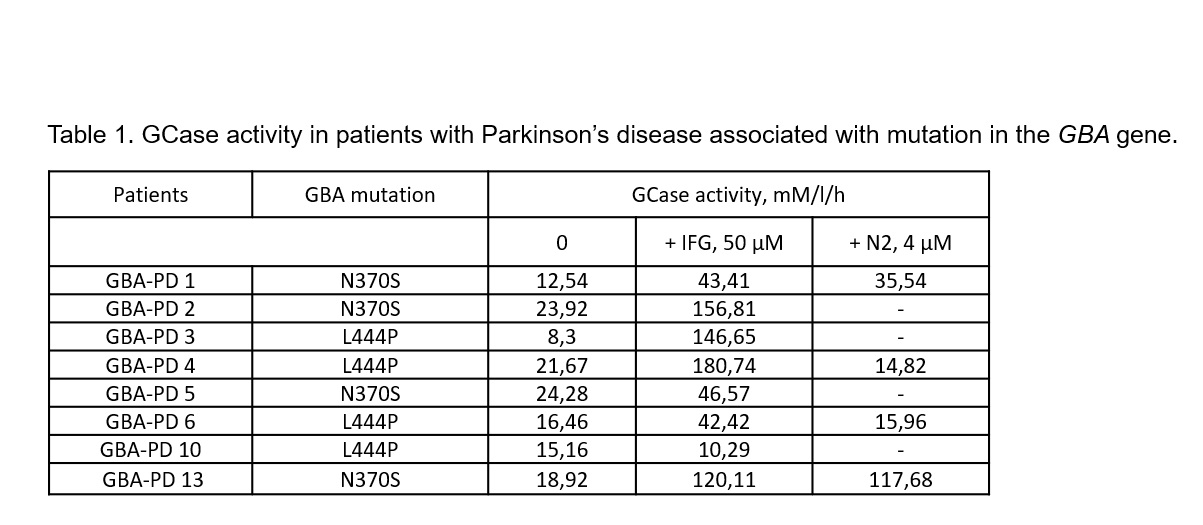
Method: Peripheral blood samples were collected from GBA-PD patients (mean age 59,1±4,0 5males) mutations (N370S, L444P). To evaluate the effect of IFG and new compounds on GCase activity, all compounds were added to cell cultures on day 4 to the final concentrations of 50 µM (IFG) and 4 µM (chemical modifications, including N2), and the cells were incubated for further 4 days. Macrophages were differentiated from purified monocytes in standard conditions. GCase activity were measured by LC-MS/MS in dried spots.
Results: We showed an increase GCase activity in macrophages from GBA-PD patients after treatment with pharmacological chaperone (IFG (47,23 (10,29-216,78)) compared to macrophages without treatment (17,71 (4,20-27,67)) p<0,001. Using molecular docking and molecular dynamics simulations we have assessed binding modes and have calculated binding free energies of chaperone N607 and its modifications in three allosteric sites of human GCase. Further we tested all compounds on macrophages. The most effective GCase activity restoration was in macrophages treated with N2 [table1].
Conclusion: N2 compound and IFG increase GCase activity in macrophages from GBA-PD patients. It is interesting to note that the effectiveness of restoring the GCase activity strongly depended on the type of GBA mutations. In the case of N370S the effectiveness in restoration of GCase activity was compatible with well-known GCase molecular chaperone IFG. Our results suggest that new potential allosteric GCase chaperone might be effective in restoration of GCase activity.The study was supported by RSF № 17-75-20159.
To cite this abstract in AMA style:
M. Nikolaev, A. Kopytova, K. Senkevich, G. Baydakova, I. Miliukhina, E. Zakharova, G. Rychkov, A. Cheblokov, F. Ibatullin, S. Pchelina, A. Emelyanov. New compound increasing glucocerebrosidase activity on primary cell cultures obtained from patients with GBA-associated Parkinson’s disease [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/new-compound-increasing-glucocerebrosidase-activity-on-primary-cell-cultures-obtained-from-patients-with-gba-associated-parkinsons-disease/. Accessed April 2, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/new-compound-increasing-glucocerebrosidase-activity-on-primary-cell-cultures-obtained-from-patients-with-gba-associated-parkinsons-disease/