Objective: To assess all-cause mortality in Medicare beneficiaries with Parkinson’s disease (PD) receiving pimavanserin or atypical antipsychotics (AAs).
Background: In PD patients, the lifetime prevalence of PD psychosis (PDP) may exceed 50% [1]. Pimavanserin, a serotonin 5HT2 antagonist, is the only drug FDA-approved for PDP. In premarketing PDP trials, there were more serious adverse events with pimavanserin than placebo, and 11% of patients died in open-label pimavanserin treatment [2]. A small observational study found numerically higher mortality with quetiapine versus pimavanserin [3], and antipsychotic drug labels warn of increased mortality in patients with dementia. Mortality with pimavanserin and AAs has not been compared in a large database.
Method: We performed a retrospective new-user cohort study of PD patients in Medicare initiating pimavanserin (n=3,227) or AAs (n=18,448) from April 2016 to March 2019. To address potential confounding, we estimated the average treatment effect in pimavanserin treated patients with inverse probability of treatment weighting (IPTW). Weighted Cox proportional hazards regression estimated adjusted hazard ratio (HR) and 95% confidence intervals (CI) for mortality. We performed a subgroup analysis comparing pimavanserin and AAs in patients with and without dementia.
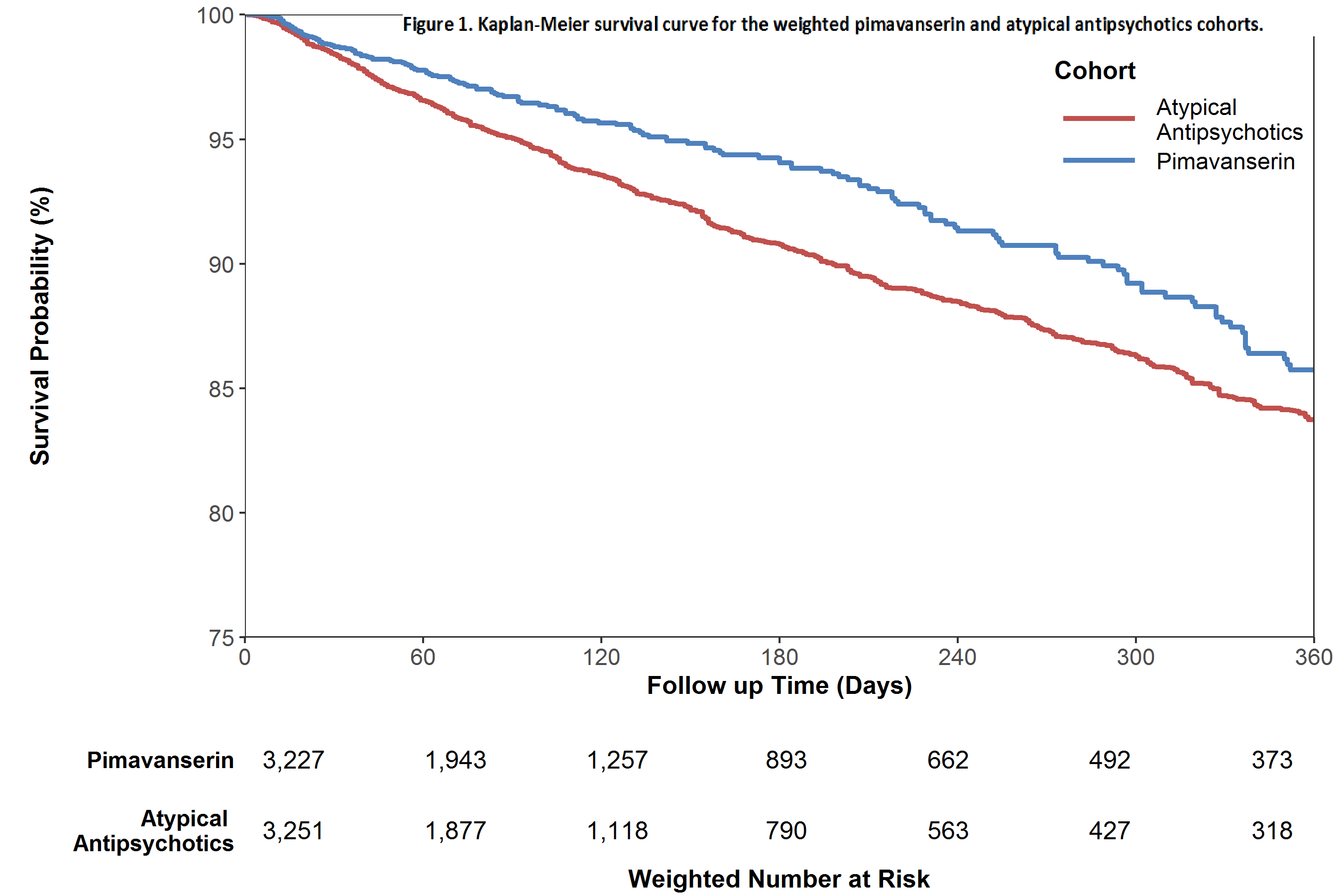
Results: Quetiapine was the chief AA (78% of users). In both groups, patients were 45% female; mean ages (years) were 78 and 79 for pimavanserin and AA users, respectively. Neurologists initiated 72% of pimavanserin and 38% of AA treatment. Before IPTW, some comorbidities were more common among AA users; after IPTW all baseline covariates were well balanced between cohorts. During follow-up 1,925 patients died. The weighted Kaplan-Meier plot [figure 1] shows higher survival probability over time for pimavanserin users. Pimavanserin use was associated with significantly lower mortality compared to AAs (HR 0.78, 95% CI 0.65-0.94). Mortality HRs in patients with (HR 0.81, 95% CI: 0.68-0.96) and without (HR 0.70, 95% CI 0.49-1.01) dementia were similar.
Conclusion: In Medicare PD patients, mortality with pimavanserin was lower than with AAs. Our results may be subject to residual confounding. This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References: [1] Fénelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci 2010; 289:12-17. [2] Mathis MV, Muoio BM, Andreason P, Avila AM, Farchione T, Atrakchi A, Temple RJ. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry 2017; 78:e668-e673. [3] Moreno GM, Gandhi R, Lessig SL, Wright B, Litvan I, Nahab FB. Mortality in patients with Parkinson disease psychosis receiving pimavanserin and quetiapine. Neurology 2018;91:797-799.
To cite this abstract in AMA style:
A. Mosholder, Y. Ma, S. Akhtar, G. Podskalny, Y. Wei, J. Liao, Y. Feng, H. Lyu, M. Wernecke, K. Leishear, L. Nelson, T. MaCurdy, J. Kelman, D. Graham. All-cause mortality in pimavanserin and atypical antipsychotic users with Parkinson’s disease in Medicare [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/all-cause-mortality-in-pimavanserin-and-atypical-antipsychotic-users-with-parkinsons-disease-in-medicare/. Accessed February 5, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/all-cause-mortality-in-pimavanserin-and-atypical-antipsychotic-users-with-parkinsons-disease-in-medicare/