Objective: This post-hoc analysis investigated the onset of treatment-emergent adverse events (TEAEs) that were considered at least possibly related to opicapone (OPC) treatment.
Background: OPC proved to be effective in treating end-of-dose motor fluctuations in Parkinson’s disease (PD) patients in two large clinical trials [1,2]. The OPTIPARK study evaluated OPC 50-mg in a heterogeneous population of PD patients treated in clinical practice.
Method: OPTIPARK was a prospective, open-label, single-arm, multicentre trial conducted in Germany (3 months) and the UK (6 months) under clinical practice conditions. PD patients with motor fluctuations received OPC 50-mg in addition to current antiparkinsonian treatment. Safety assessments included evaluation of TEAEs.
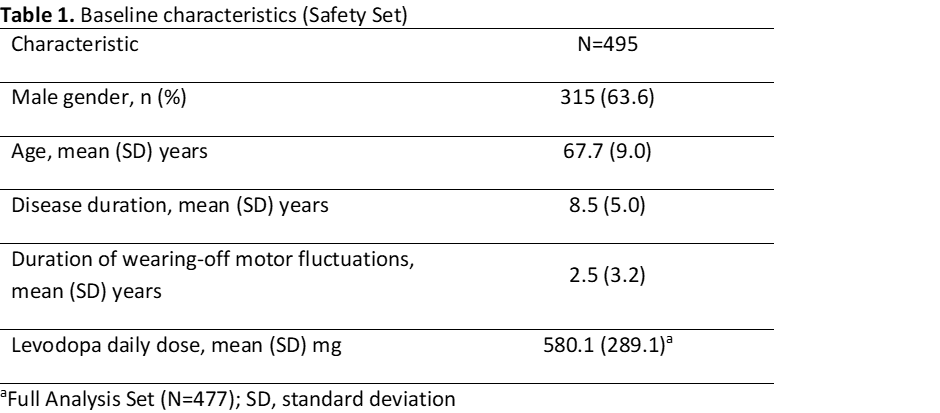
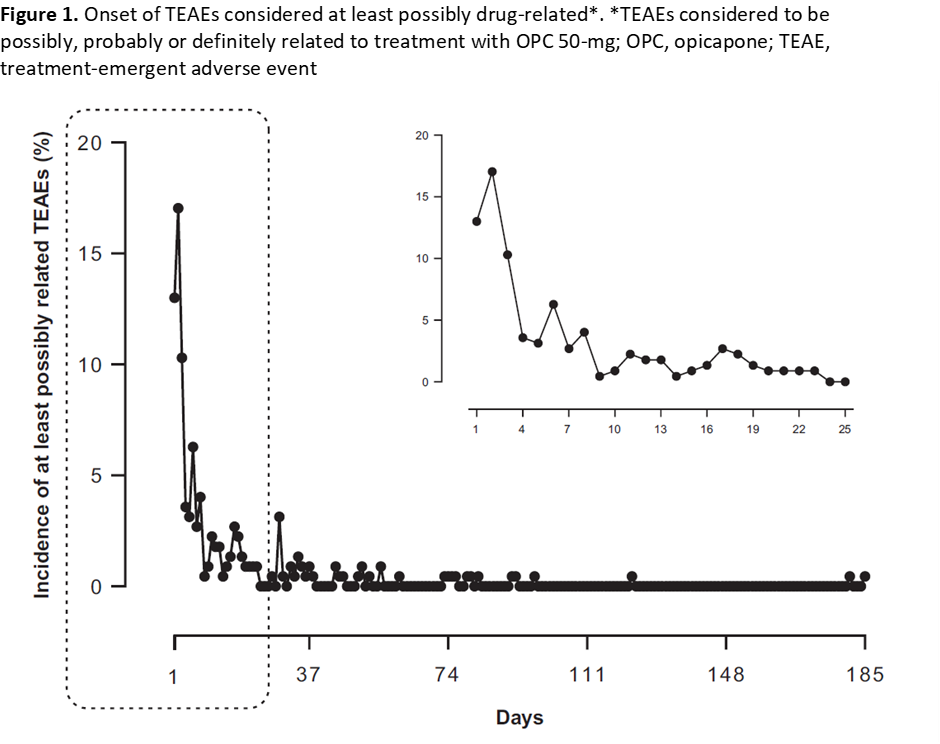
Results: Overall, 495 patients took >=1 dose of OPC (Safety Set; Table 1), of whom 223 (45.1%) reported TEAEs considered at least possibly drug-related. The majority of at least possibly drug-related TEAEs were reported during the first week of OPC treatment, and from the third week onwards the incidence of these TEAEs was consistently low (<4%) (Figure 1). Within the first week of OPC treatment, dyskinesia was the most frequently reported at least possibly drug-related TEAE (6.5%) but with very low impact on patient discontinuation (<0.5%) (Figure 2).
Conclusion: In the OPTIPARK study, most TEAEs considered at least possibly drug-related had fast onset within the first week of OPC treatment followed by consistently low incidence for 6 months. These observations are relevant for patient management, namely concerning levodopa adjustment, in clinical practice.
References: 1. Ferreira et al., Lancet Neurol. 2016;15(2):154-165. 2. Lees et al., JAMA Neurol. 2017;74(2):197-206.
To cite this abstract in AMA style:
A. Lees, H. Reichmann, J.F Rocha, D. Magalhães, P. Soares-da-Silva. Onset of Drug-Related Adverse Events in Parkinson’s Disease Patients with Motor Fluctuations Treated with Opicapone in Clinical Practice: OPTIPARK Post-Hoc Analysis [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/onset-of-drug-related-adverse-events-in-parkinsons-disease-patients-with-motor-fluctuations-treated-with-opicapone-in-clinical-practice-optipark-post-hoc-analysis/. Accessed December 18, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/onset-of-drug-related-adverse-events-in-parkinsons-disease-patients-with-motor-fluctuations-treated-with-opicapone-in-clinical-practice-optipark-post-hoc-analysis/