Category: Parkinson’s Disease: Clinical Trials
Objective: To study the safety and efficacy of transcutaneous magnetic spinal cord stimulation (SCS) on freezing of gait (FoG) and other motor symptoms in a cohort of PD patients.
Background: The current treatment of FoG in PD remains partially efficacious (1), probably reflecting its multifaced underlying pathophysiology (2). A positive effect of epidural SCS on locomotive activity has been reported in few PD patients (3). Transcutaneous SCS is an emerging method that activates similar target neural structures noninvasively (4).
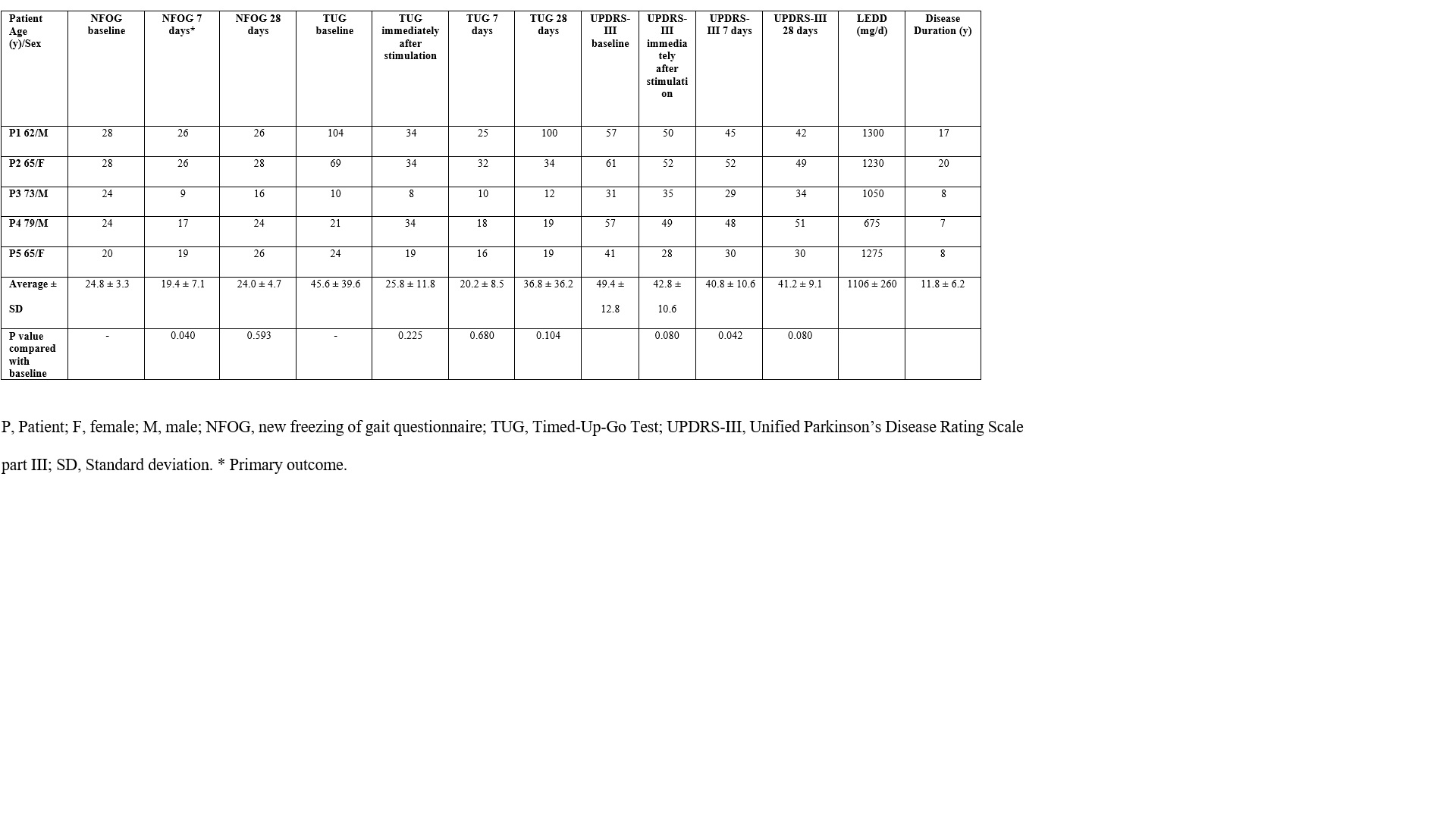
Method: Five patients were included in this prospective, open-label, pilot study. PD patients with moderate FoG (UPDRS part III Fog subscore of 2 or greater) as the most bothersome symptom despite optimized treatment were included. The protocol was applied to patients during off-medication state. A trial of transcutaneous magnetic SCS was performed with a figure-of-eight coil (Magventure® MagPro® R20) at the level of the fifth thoracic vertebra. The stimulation intensity was set at 90% of motor threshold (fifth thoracic nerves contraction). Each session consisted of 20 theta-burst trains at 5 Hz, with 20 pulses each at 50 Hz, for a total of 400 pulses throughout 2 min. Measurements were recorded at baseline, immediately after the stimulation session, 7 days after stimulation, and 28 days after stimulation.
Results: The patients revealed improvement in FoG, gait speed, and other motor symptoms 7 days after SCS, which tended to return to baseline scores at the final evaluation (28th day, p > 0.05). Seven days after stimulation, new freezing of gait questionnaire improved by 22% (p =0.40) and UPDRS-III by 17.4% (p = 0.042) compared with baseline. Gait speed (timed up and go, TUG) was numerical – but not significantly – better after stimulation (improvement in TUG by 48.2%, p = 0.06). Patients’ global impression of change was ‘much improved’ for four patients and ‘minimally improved’ for one patient.
Conclusion: In this pilot trial, transcutaneous magnetic SCS improved gait parameters in patients with PD, with no severe side effects. The improvement was reversible, since there was no carryover effect 28 days after stimulation. To our knowledge, this is the first study to report a novel paradigm of stimulating the spinal cord – a ‘highway’ of sensory information largely connected with supraspinal gait circuits – noninvasively through a transcutaneous magnetic field. Phase II trials are needed.
References: 1.Gilat M, Lígia Silva de Lima A, Bloem BR, Shine JM, Nonnekes J, Lewis SJG. Freezing of gait: Promising avenues for future treatment. Parkinsonism Relat Disord. 2018;52:7–16. 2. Weiss D, Schoellmann A, Fox MD, Bohnen NI, Factor SA, Nieuwboer A, et al. Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain. 2019 Oct 24; 3. Yadav AP, Nicolelis MAL. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov Disord. 2017 Jun;32(6):820–32. 4. Hofstoetter US, Freundl B, Danner SM, Krenn MJ, Mayr W, Binder H, et al. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. Neurotrauma. 2019 Aug 9;
To cite this abstract in AMA style:
J.R Menezes, R.B Carra, G.A Nunes, J.S Simões, M.J Teixeira, K.P Duarte, D.C Andrade, E.R Barbosa, M.A Marcolin, R.G Cury. Transcutaneous magnetic spinal cord stimulation for freezing of gait in Parkinson’s disease (PD) [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/transcutaneous-magnetic-spinal-cord-stimulation-for-freezing-of-gait-in-parkinsons-disease-pd/. Accessed February 6, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/transcutaneous-magnetic-spinal-cord-stimulation-for-freezing-of-gait-in-parkinsons-disease-pd/