Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the effect of safinamide treatment on non-motor and motor experiences of daily living as assessed using the MDS-UPDRS (Parts I nm-EDLand II m-EDL).
Background: Safinamide is an a-aminoamide with both dopaminergic and non-dopaminergic (glutamatergic) mechanisms of action. While the efficacy of safinamide in improving ON time has been established, its effectiveness on non-motor symptoms is less clear. In one longitudinal study, MDS-UPDRS Part I progressed by 0.42 points per year, and Part II by 0.8 points per year.1
Method: The ProGo study evaluated PD patients (30-80 years) with motor fluctuations prescribed safinamide at a routine clinic visit who subsequently gave informed consent to participate in this open label study. Primary endpoint was MDS-UPDRS Parts I and II at day 60; patients could continue in an optional 4-month extension (NCT03944785). Patients enrolled were either naïve to previous MAO-B inhibitor treatment or switched from another MAO-B inhibitor or a dopamine agonist. The MDS-UPDRS Parts I and II were assessed at baseline and Day 60.
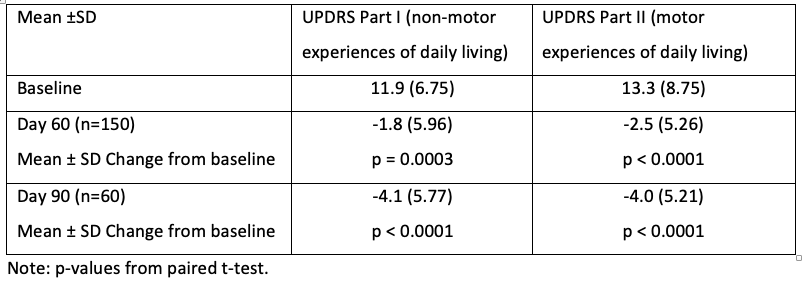
Results: Of the 165 patients enrolled, 164 subjects were in the safety population and 153 in the evaluable population. Mean ±SD levodopa dose was 714 ±555 mg; 78% (n=128) were naïve to MAO-B inhibition, 21% (n=34) switched from a prior MAO-B inhibitor and 1% (n=2) from a dopamine agonist. At Day 60, MDS-UPDRS Part I and Part II subscores were reduced from baseline [Table1]. Patients who continued in the extension phase experienced greater improvement.
Conclusion: This prospective open label study provides evidence for nonmotor symptom improvement in PD patients with motor fluctuations prescribed safinamide. Motor experiences of daily living were also improved. Both nonmotor and motor improvement may reflect safinamide’s dual dopaminergic and non dopaminergic mechanisms of action.
References: Poewe et al. Mov Disord. 2015; 30(4):589-92.
To cite this abstract in AMA style:
S. Isaacson, T. Clinch, E. Farbman, R. Pahwa. Impact of safinamide on non-motor and motor experiences of daily living: Primary results from the prospective, observational ProGo study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/impact-of-safinamide-on-non-motor-and-motor-experiences-of-daily-living-primary-results-from-the-prospective-observational-progo-study/. Accessed December 25, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-safinamide-on-non-motor-and-motor-experiences-of-daily-living-primary-results-from-the-prospective-observational-progo-study/