Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the effect of BL-dyskinesia on the efficacy and safety of istradefylline in 4 randomized, placebo-controlled, double-blind, phase 2b/3 trials.
Background: Istradefylline, a selective adenosine A2A receptor antagonist that acts via the indirect basal ganglia outflow pathway, is indicated in the US as adjunctive treatment to levodopa/carbidopa in adults with PD experiencing OFF episodes. Dyskinesia was the most frequently reported adverse event (AE). This post hoc analysis included the 4 trials that were the basis for the US Food and Drug Administration’s approval of istradefylline.
Method: In this pooled analysis, patients received istradefylline (20 or 40mg/day) or placebo for 12 weeks. Change from baseline in OFF time (istradefylline vs placebo) provided the primary endpoint and was derived from patient-completed 24-hour ON/OFF diaries. Subgroup analyses were conducted on patients with (+BL-dyskinesia) and without (–BL-dyskinesia) BL-dyskinesia, as identified in the patient-completed baseline ON/OFF diaries. AEs were recorded throughout as spontaneous reports.
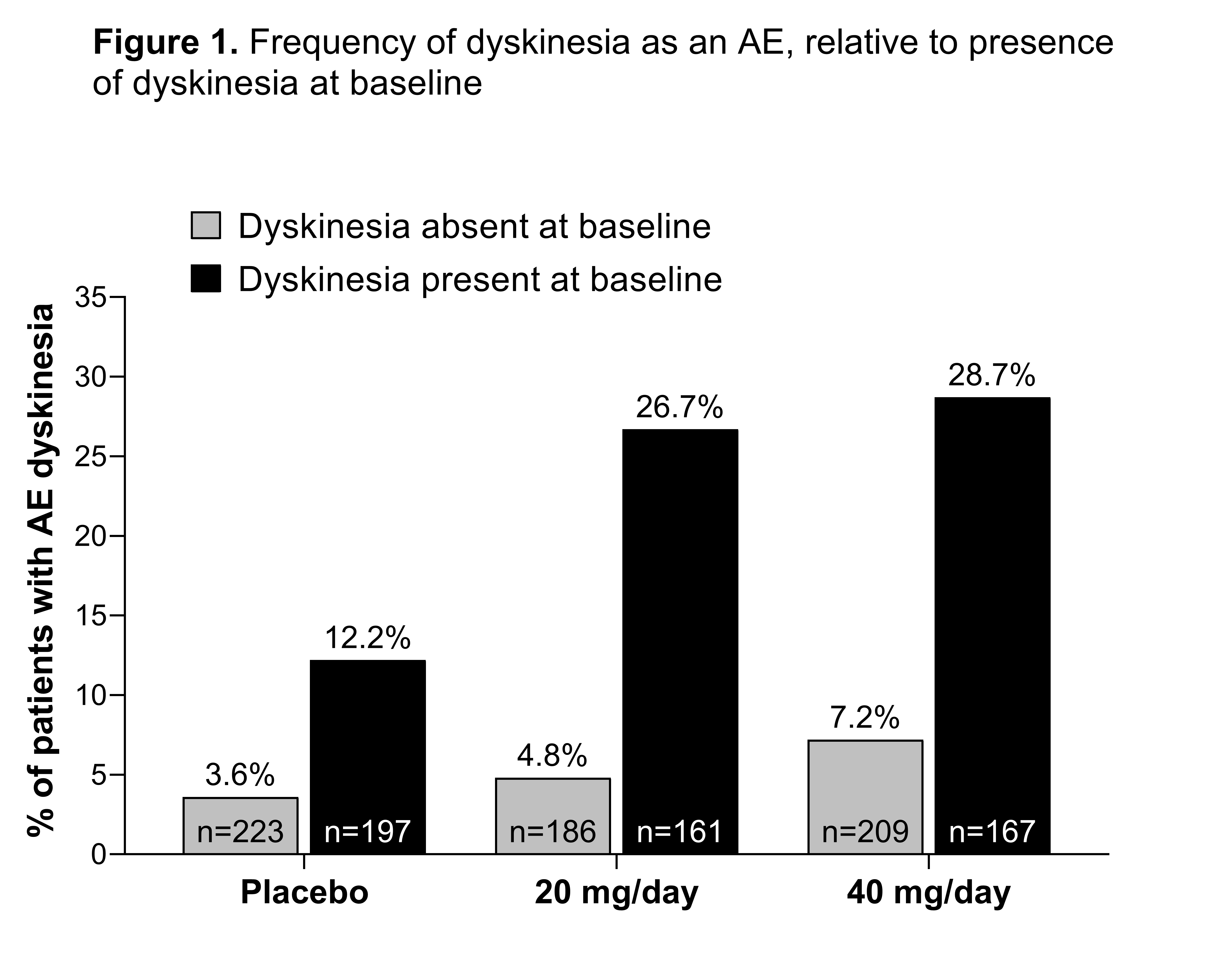
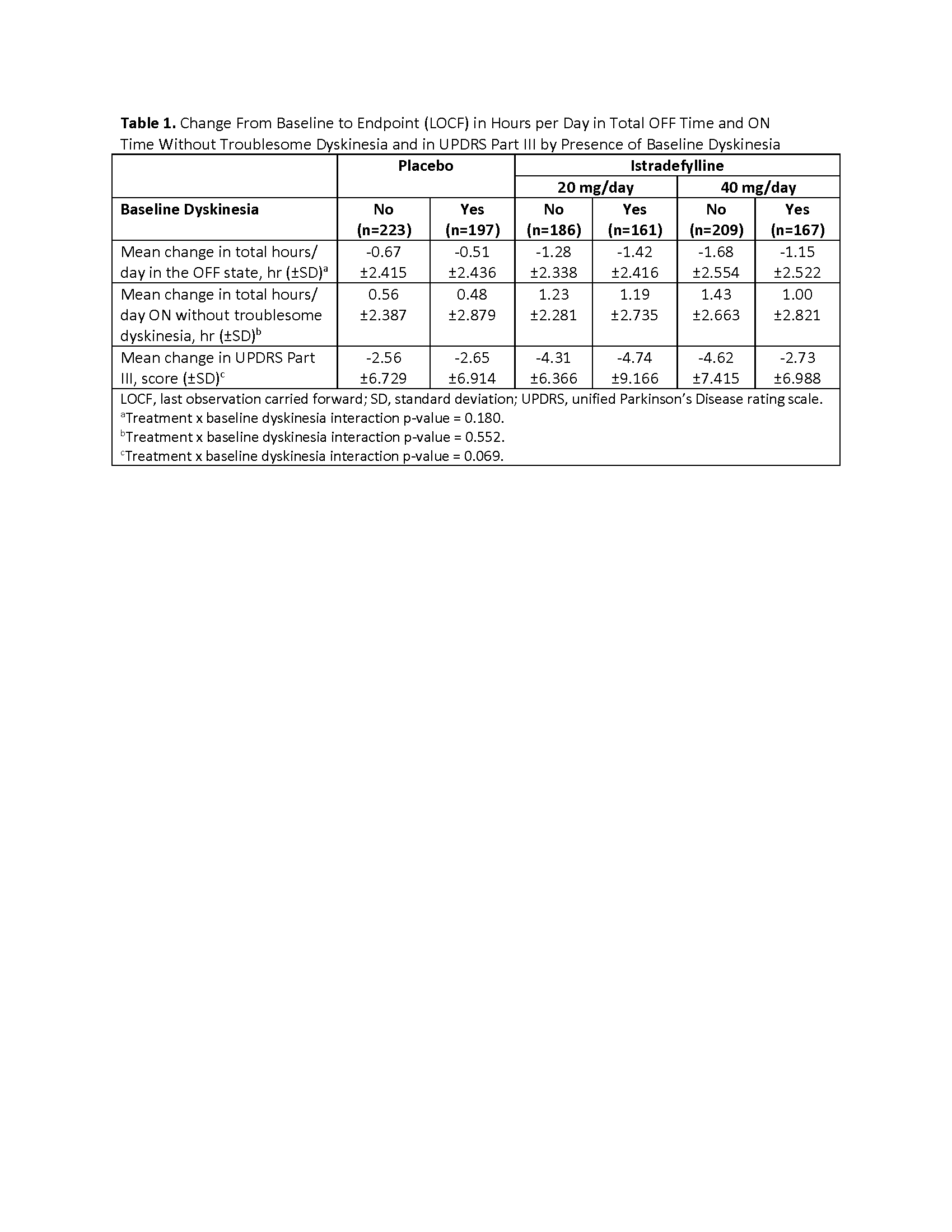
Results: 1143 patients (+BL-dyskinesia, n=525; –BL-dyskinesia, n=618) were included. Baseline characteristics and demographics were generally similar between subgroups, with the exception of a higher baseline daily levodopa dosage and longer time since PD diagnosis in the +BL-dyskinesia vs –BL-dyskinesia subgroups. Dyskinesia AEs occurred more frequently with istradefylline vs placebo in both the +BL-dyskinesia and –BL-dyskinesia subgroups [figure1]. In patients treated with either dose of istradefylline, mean reduction in OFF time and increase in ON time without troublesome dyskinesia (ON-WOTD) were greater than with placebo; these changes were unaffected by the presence of baseline dyskinesia. In the 40 mg/day treatment arm, the –BL-dyskinesia subgroup had a greater mean decrease in UPDRS III scores than the +BL-dyskinesia subgroup [table1]. Istradefylline was well tolerated.
Conclusion: The frequency of dyskinesia as an AE was greater with istradefylline treatment vs placebo, although infrequently reported in the –BL-dyskinesia subgroup. The presence of baseline dyskinesia had little effect on istradefylline-induced improvements in OFF time and ON-WOTD time.
To cite this abstract in AMA style:
L. Elmer, K. Toyama, J. Parno, D. Braccia, R. Ristuccia, A. Mori. Safety and efficacy of istradefylline, an adenosine A2A receptor antagonist, as a function of baseline dyskinesia (BL-dyskinesia) in Parkinson’s disease (PD): A pooled analysis of 4 studies [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-istradefylline-an-adenosine-a2a-receptor-antagonist-as-a-function-of-baseline-dyskinesia-bl-dyskinesia-in-parkinsons-disease-pd-a-pooled-analysis-of-4-studies/. Accessed March 6, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-istradefylline-an-adenosine-a2a-receptor-antagonist-as-a-function-of-baseline-dyskinesia-bl-dyskinesia-in-parkinsons-disease-pd-a-pooled-analysis-of-4-studies/