Category: Parkinson's Disease: Pathophysiology
Objective: To evaluate the sensitivity of instrumented measures of motor dysfunction and candidate serological markers of disease to predict disease progression in de novo Parkinson’s disease patients compared with the current standard of clinical progression, the motor subscore of UPDRS part III.
Background: One of the greatest barriers to successful translation of preclinical findings to human clinical trials in Parkinson’s disease (PD) has been the lack of reliable, cost-effective, and sensitive biomarkers of early PD progression. The motor subscore of the modified Unified Parkinson’s Disease Rating Scale (UPDRS Part 3) is the most common functional endpoint used in PD trials but it has limited sensitivity to change in early PD. The motor measures in the NIH toolbox represent an alternative to UPDRS, but those measures have not been evaluated in early untreated PD. Previous studies have also identified serological markers that are increased in PD patients compared to controls, including the oxidative stress biomarker Nrf2, but the relationship between NRF2 and disease progression in early PD has not been evaluated.
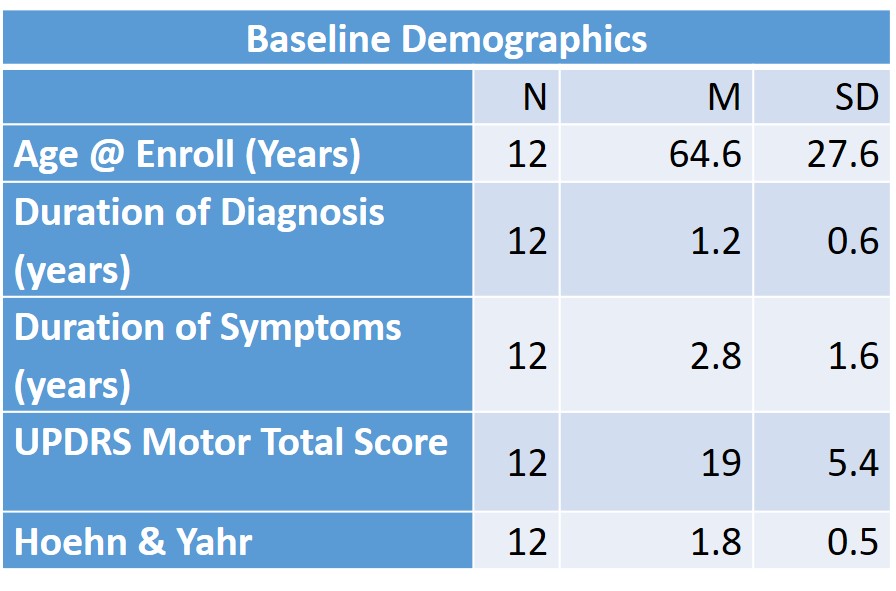
Method: Patients with de novo PD were enrolled and followed for 6 months. At each visit, a movement disorder specialist administered the UPDRS part III and then collected NIH toolbox measures: time to complete 9-hole pegboard test, grip strength, standing balance, gait speed, and finger tapping. Blood samples were drawn on each patient, PBMCs were isolated and Nrf2 expression determined using qPCR.
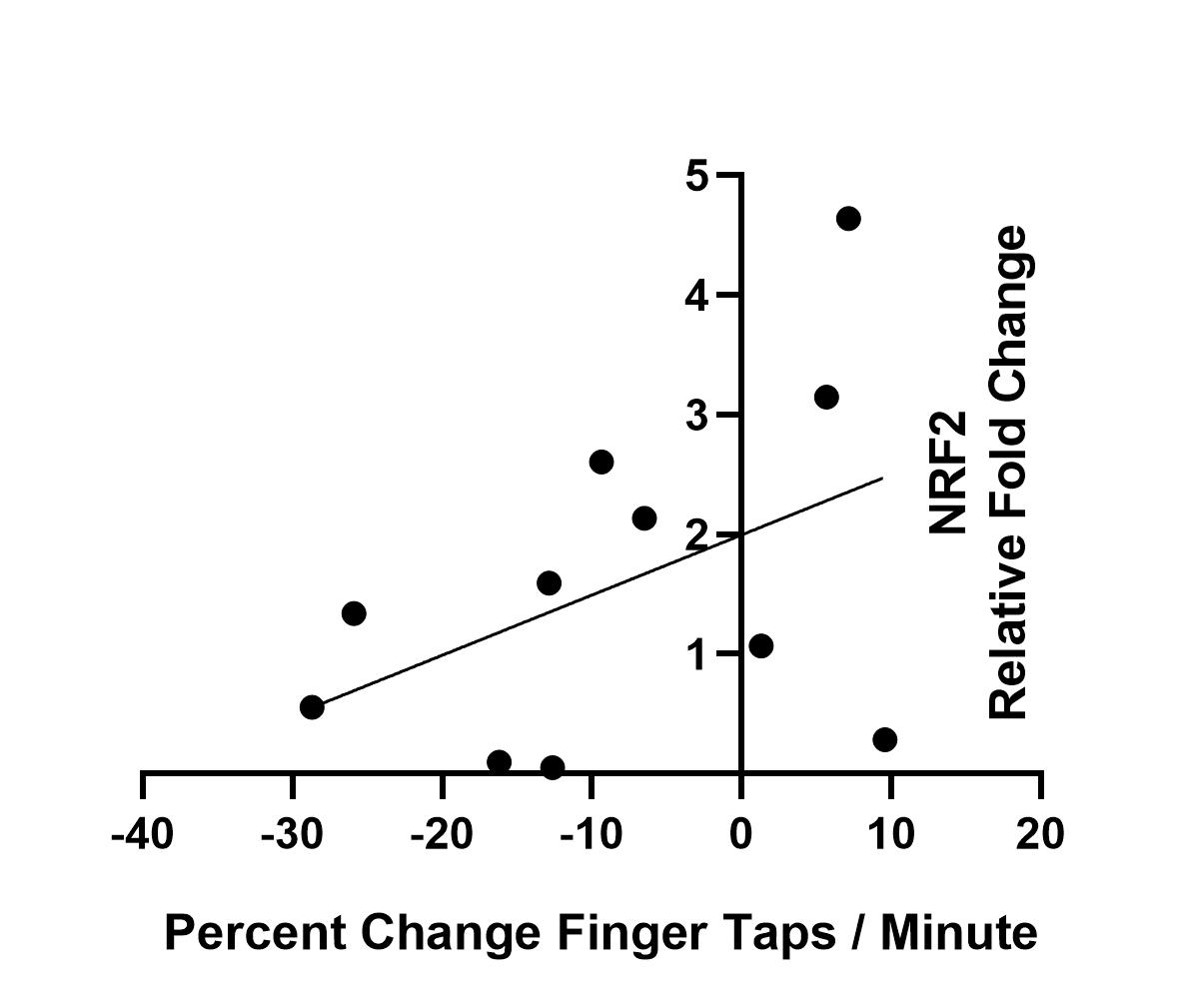
Results: Twelve patients completed both visits, and at 6 months, there was no significant change in UPDRS, but the NIH toolbox finger-tapping score distinguished “progressors” from “non-progressors” (mean change in finger taps per minute -8.5 and 24.75, respectively, p=0.014). There was also a trend toward a correlation between worsening finger tap scores and PBMC Nrf2 expression (p=0.16).
Conclusion: Our findings suggest that objective motor measures, including finger tap scores, and serological measures of Nrf2 expression exhibit changes in patients earlier than changes in UPDRS scores can be reliably observed. Longer-term assessment of this patient cohort will allow us to determine whether these changes in objective motor tests and Nrf2 expression predict differential rates of disease progression.
To cite this abstract in AMA style:
L. Neilson, J. Baird, B. Lobb, H. Vanderjagt, N. Gray, S. O'Connor, J. Quinn. Identifying biomarkers to predict disease progression in early stage Parkinson’s disease patients [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/identifying-biomarkers-to-predict-disease-progression-in-early-stage-parkinsons-disease-patients/. Accessed January 5, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/identifying-biomarkers-to-predict-disease-progression-in-early-stage-parkinsons-disease-patients/