Category: Parkinson's Disease: Neuroimaging
Objective: To explore the pathogenesis of dyskinesia, the present study was conducted to investigate that how large-scale functional network interactions change dynamically in the temporal domain of Parkinson’s disease (PD) patients with and without levodopa-induced dyskinesia (LID).
Background: LID is one disabling motor complication of chronic levodopa therapy in patients with PD. The precise pathophysiological mechanisms underlying this motor disorder remain largely unclear. Traditional resting state functional connectivity on levodopa-induced dyskinesia is measured with the assumption that intrinsic fluctuations during the MRI scan are stationary. Dynamic resting state functional network connectivity have recently been proposed to capture temporal variations of functional network connectivity throughout MRI scan.
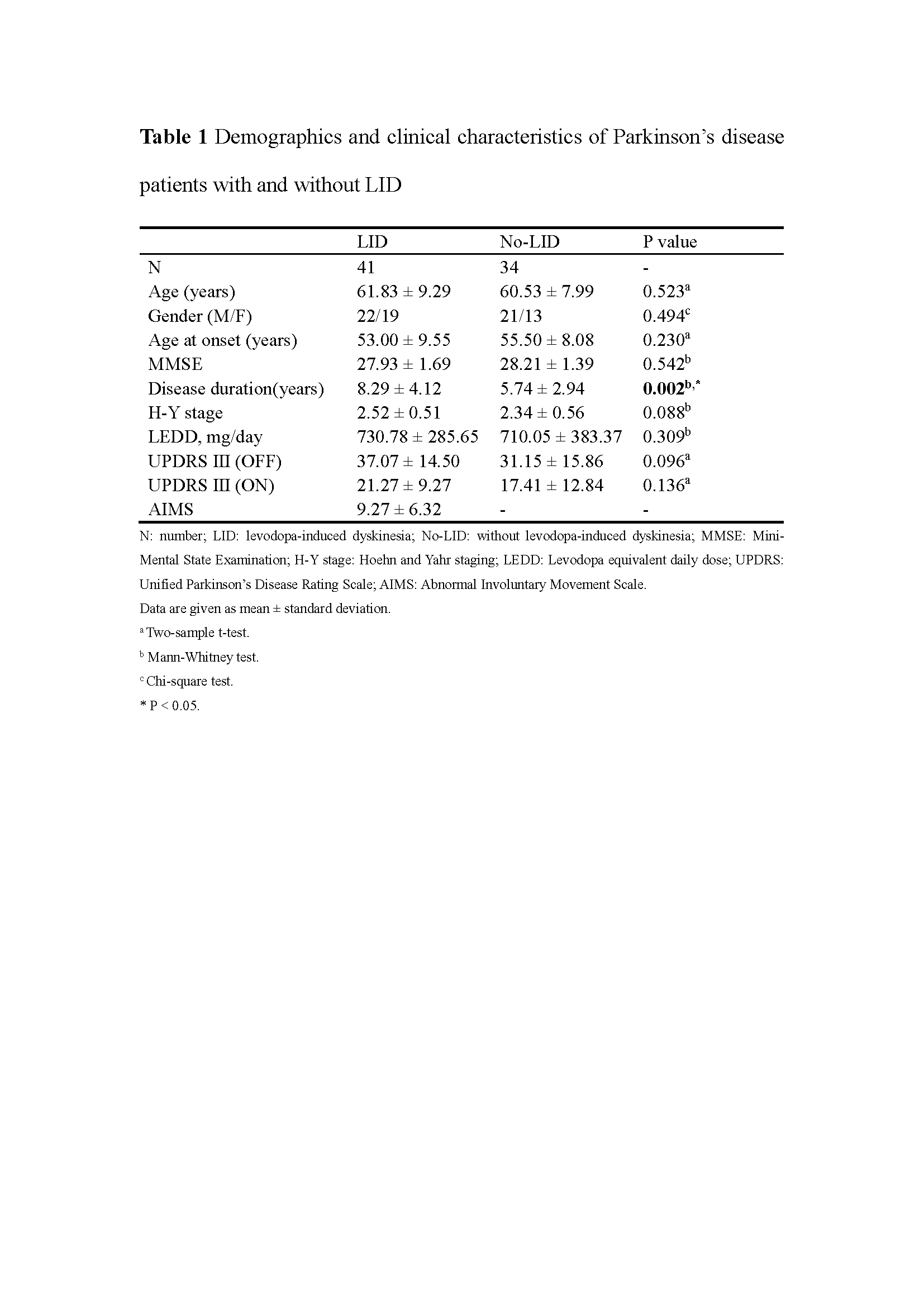
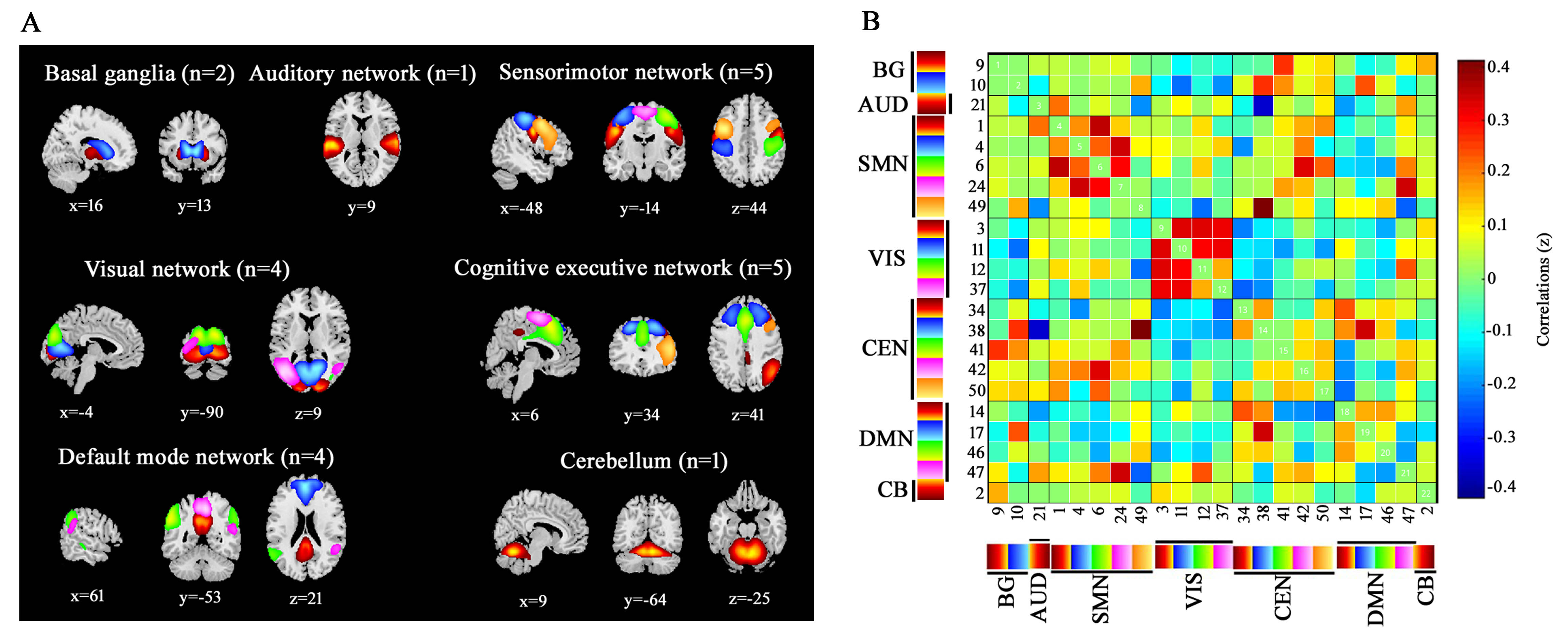
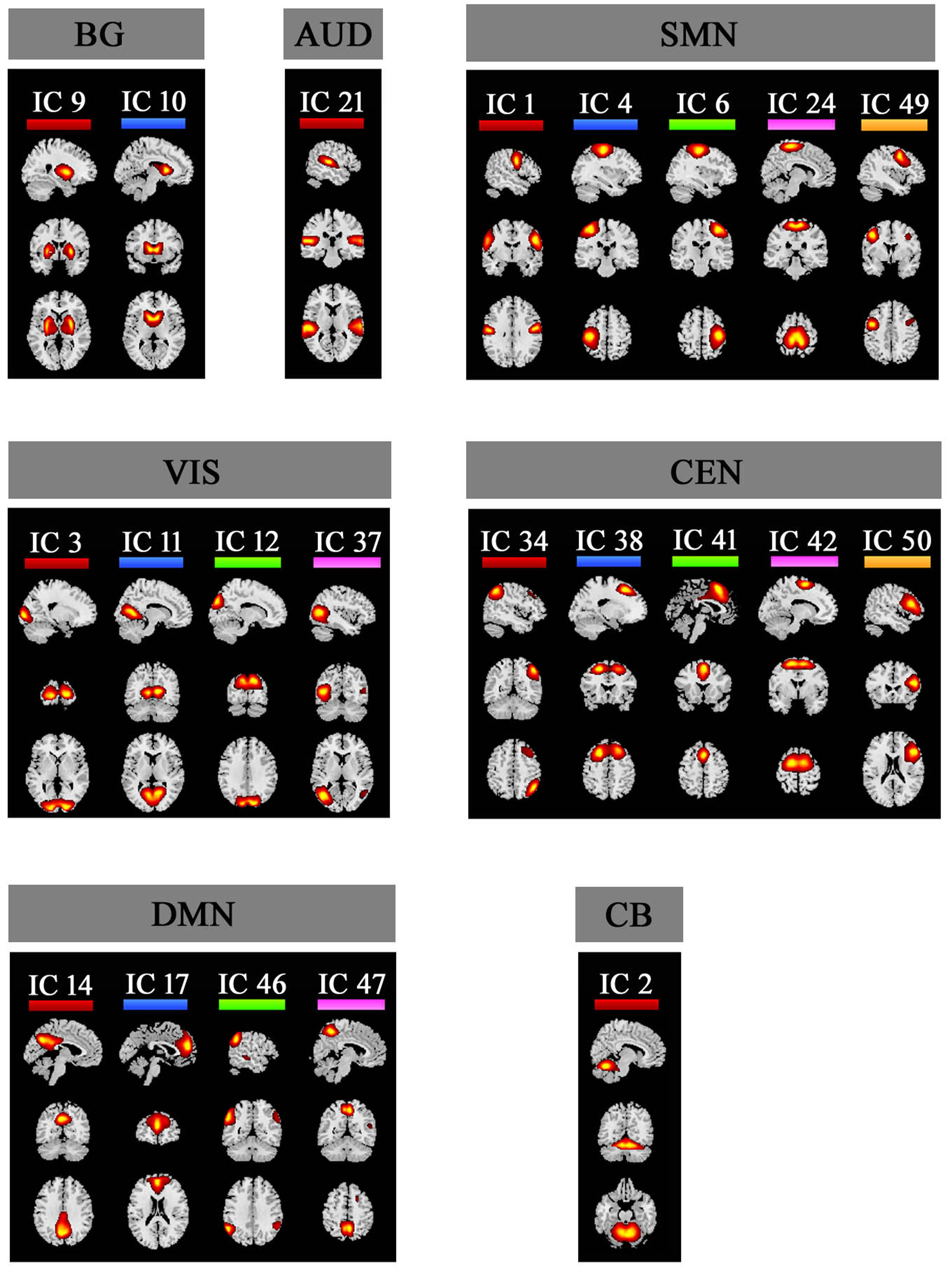
Method: We evaluated 41 PD patients with LID (LID group) and 34 clinically matched PD patients without LID (No-LID group) using dynamic functional network connectivity approach, on and off their levodopa medication. Group spatial independent component analysis, sliding-window approach followed by k-means clusters were used to study the time-varying resting state functional MRI.
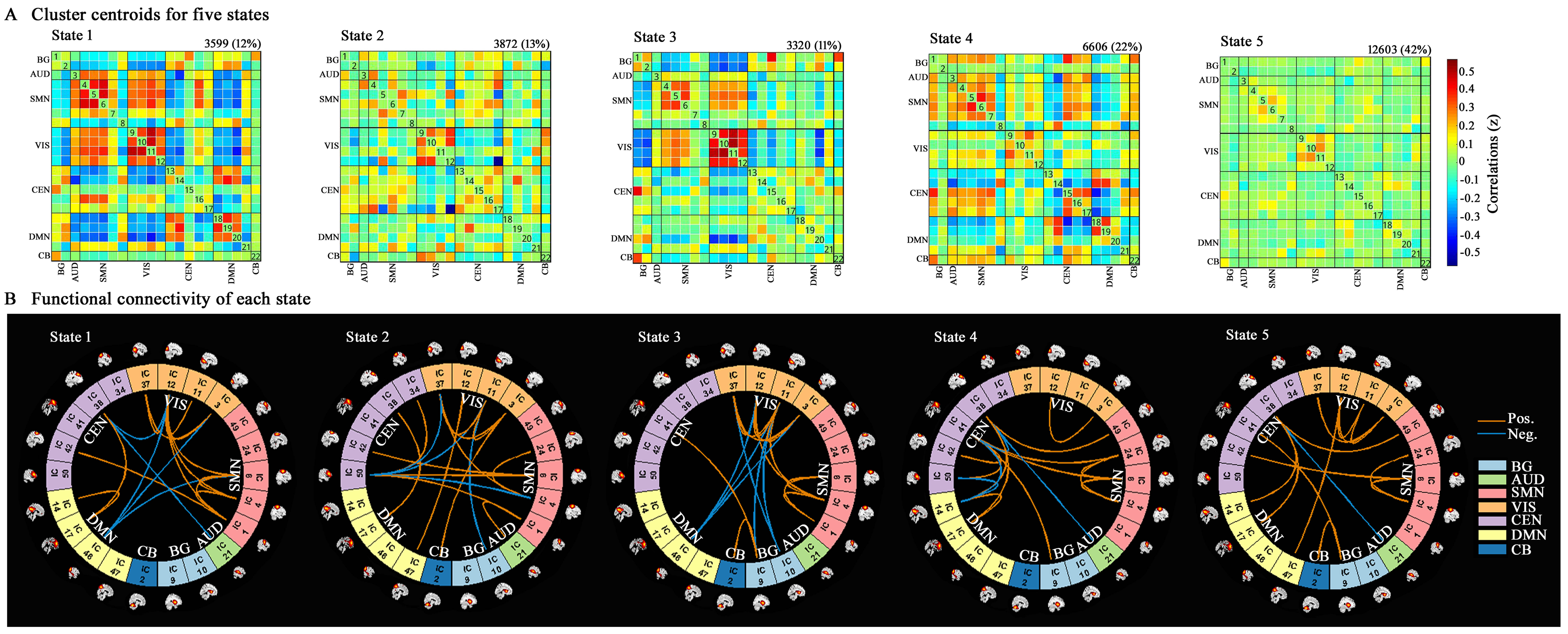
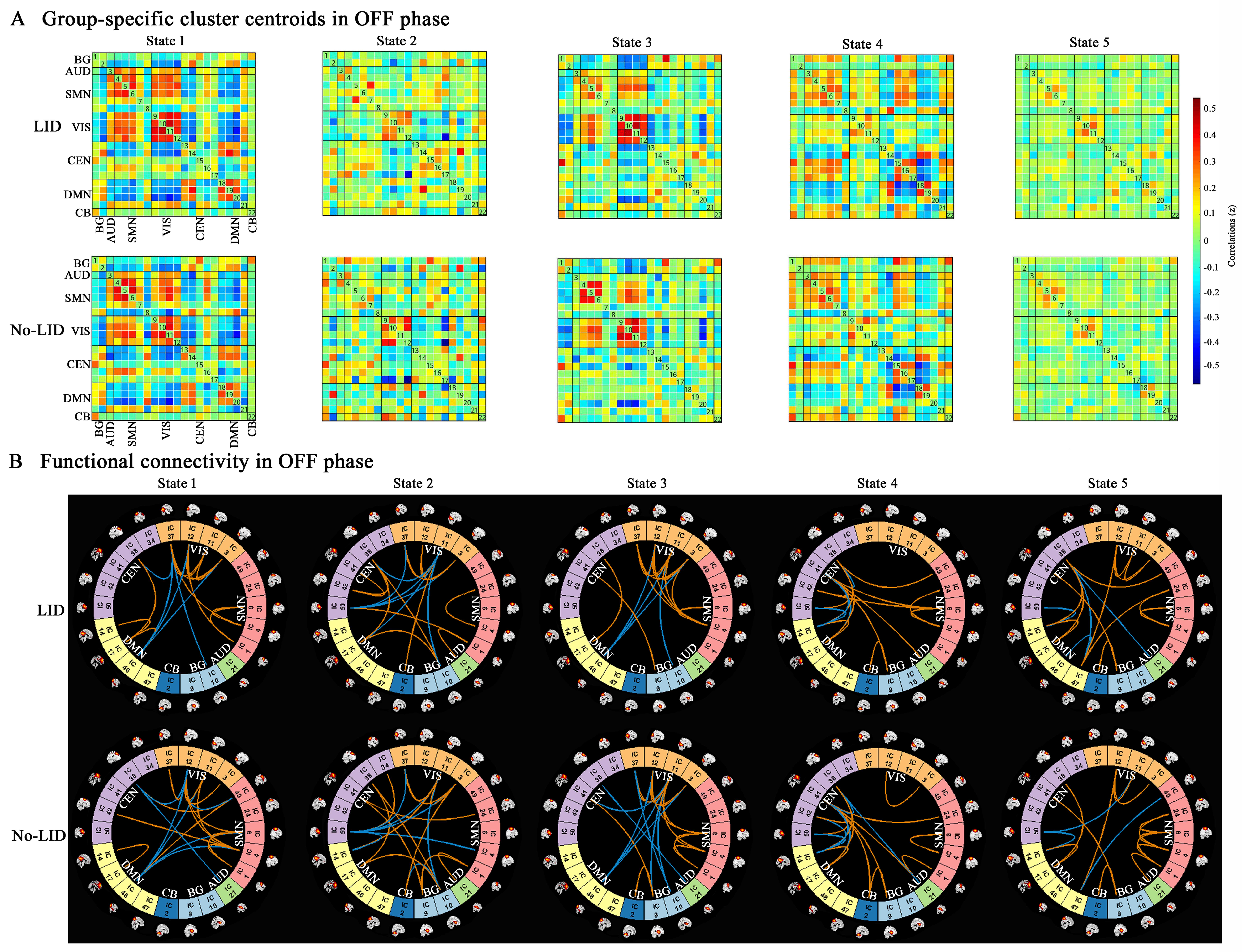
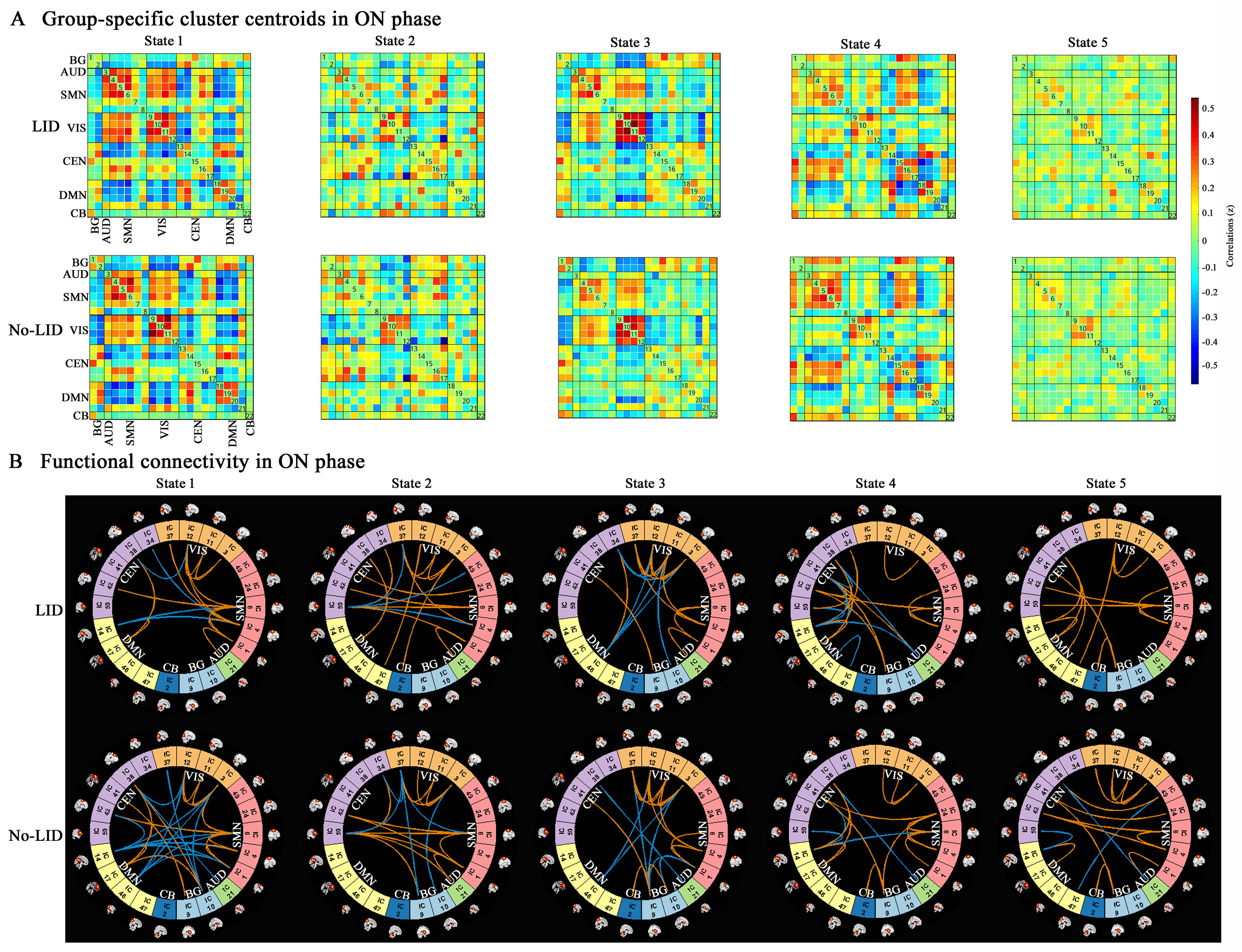
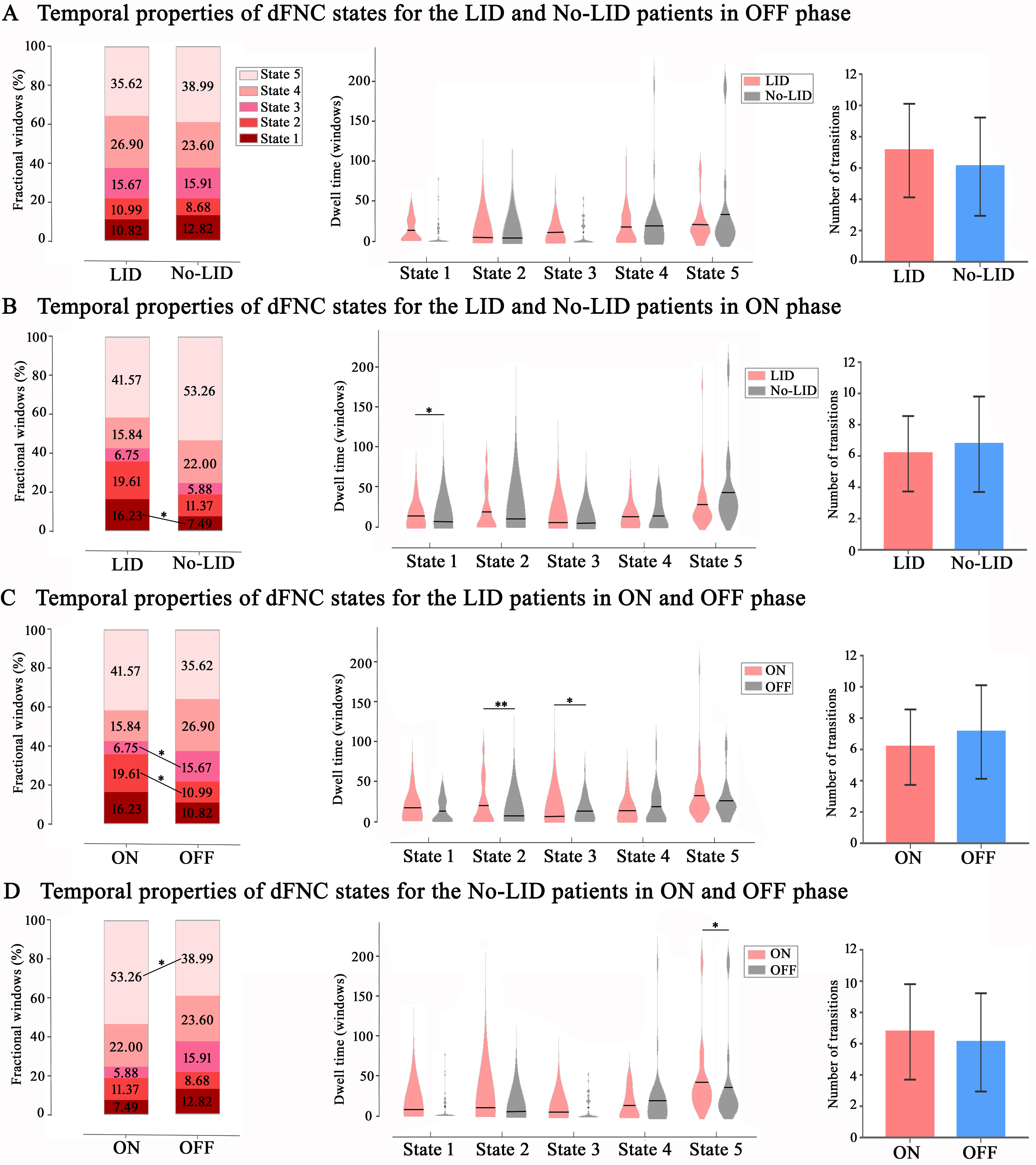
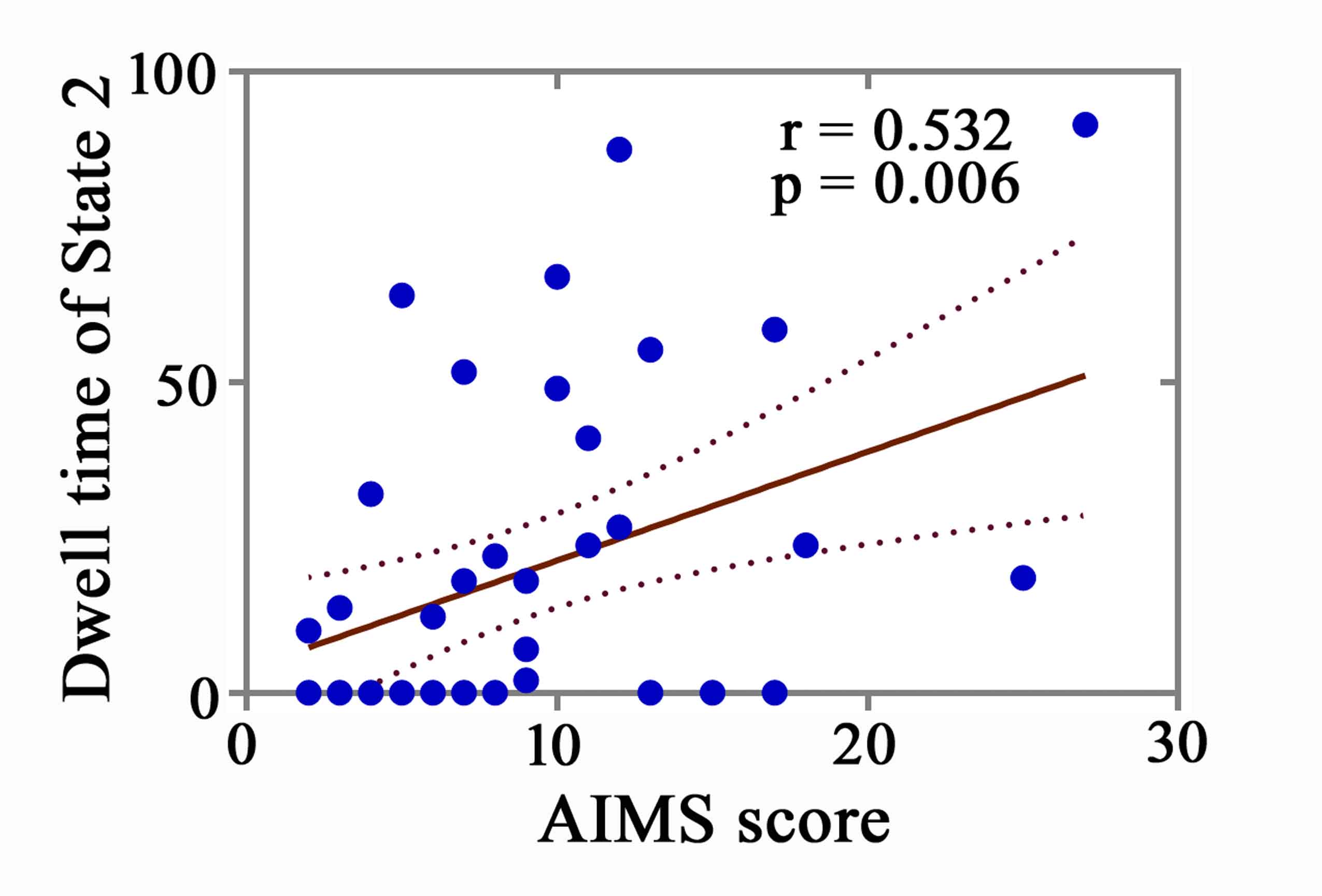
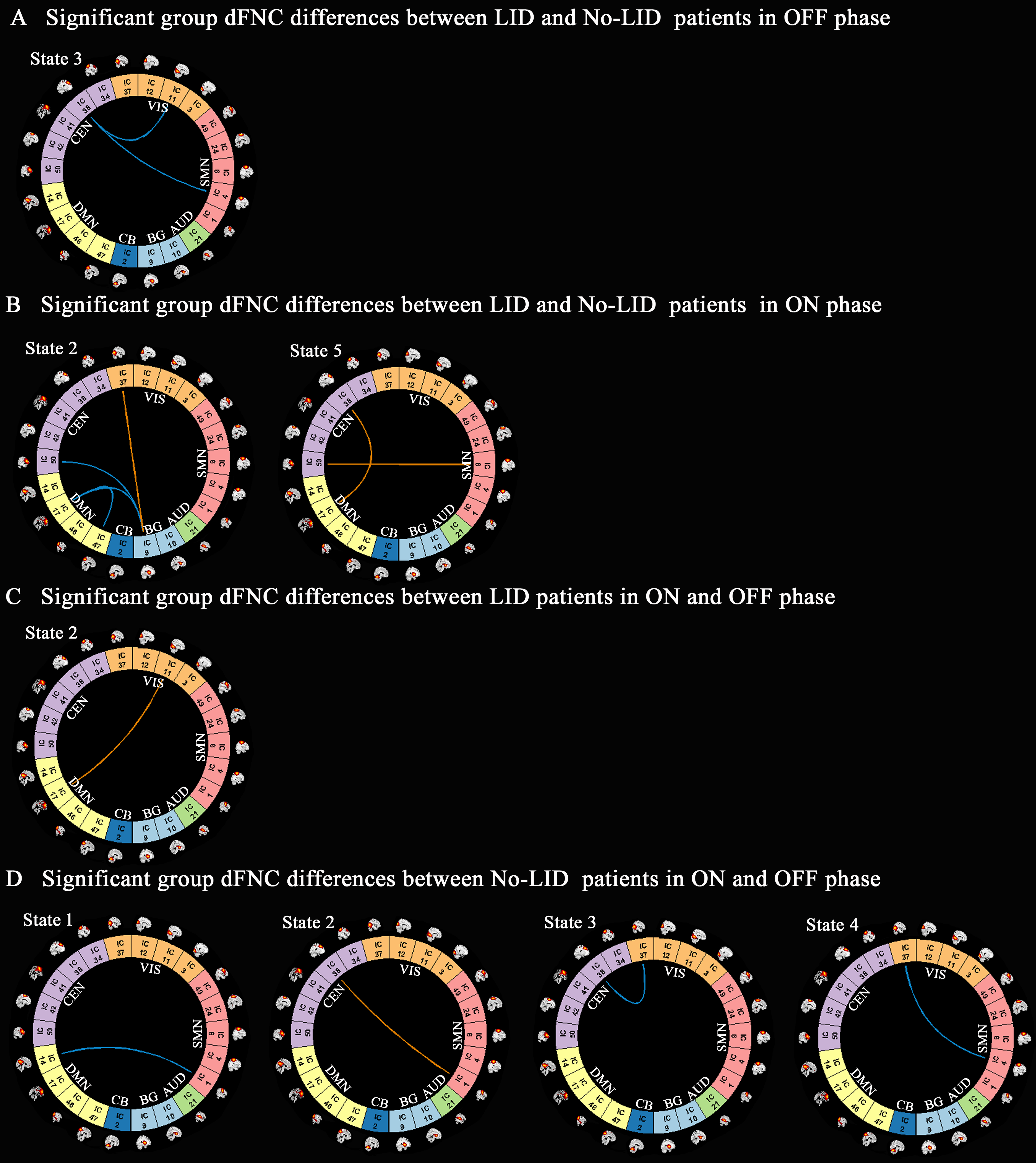
Results: The dynamic analysis identified seven networks configured into five discrete dynamic brain states: four less frequent and strongly inter-network connected, State 1-4, and a more frequent, relatively sparsely and weakly intra-network connected, State 5. At OFF phase, no significant differences of fractional windows and dwell time were found in PD patients with and without LID. At ON phase, State 1 occurred more frequently and dwelled longer in LID group compared than No-LID group. When switching from OFF to ON phase, LID group occurred more frequently and dwelled longer in State 2 and occurred less frequently and dwelled shorter in State 3, while No-LID group occurred more often and dwelled longer in State 5. Additionally, correlation analysis demonstrates that more severe dyskinesia correlates with more time spent in State 2 during ON phase.
Conclusion: The present study indicates that more severe dyskinesia was associated with prolonged time spent in in a state dominated by strong interconnections between cognitive executive network and sensorimotor network, visual network, centred on inferior frontal cortex in cognitive executive network.
References: 1. Cerasa A, Koch G, Donzuso G, et al. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain 2015;138:414-427. 2. Cerasa A, Donzuso G, Morelli M, et al. The motor inhibition system in Parkinson’s disease with levodopa-induced dyskinesias. Mov Disord 2015;30:1912-1920. 3. Herz DM, Haagensen BN, Christensen MS, et al. Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans. Brain 2015;138:1658-1666. 4. Cerasa A, Morelli M, Augimeri A, et al. Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 2013;19:123-125. 5. Cerasa A, Messina D, Pugliese P, et al. Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord 2011;26:807-812. 6. Herz DM, Haagensen BN, Nielsen SH, Madsen KH, Lokkegaard A, Siebner HR. Resting-state connectivity predicts levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord 2016;31:521-529. 7. Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 2014;24:663-676. 8. Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 2013;34:2154-2177. 9. Calhoun VD, Miller R, Pearlson G, Adalı T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 2014;84:262-274. 10. Kim J, Criaud M, Cho SS, et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 2017;140:2955-2967. 11. Fiorenzato E, Strafella AP, Kim J, et al. Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain 2019; 142: 2860-2872. 12. Tu Y, Fu Z, Zeng F, et al. Abnormal thalamocortical network dynamics in migraine. Neurology 2019;92:e2706-e2716. 13. Liu F, Wang Y, Li M, et al. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp 2017;38:957-973. 14. Guo S, Zhao W, Tao H, Liu Z, Palaniyappan L. The instability of functional connectivity in patients with schizophrenia and their siblings: A dynamic connectivity study. Schizophr Res 2018;195:183-189. 15. Schumacher J, Peraza LR, Firbank M, et al. Dynamic functional connectivity changes in dementia with Lewy bodies and Alzheimer’s disease. NeuroImage: Clinical 2019;22:101812. 16. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181-184. 17. Cerasa A, Salsone M, Morelli M, et al. Age at onset influences neurodegenerative processes underlying PD with levodopa-induced dyskinesias. Parkinsonism Relat Disord 2013;19:883-888. 18. Pavese N, Evans AH, Tai YF, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology 2006;67:1612-1617. 19. Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage 2013;80:360-378. 20. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012;59:2142-2154. 21. Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 1995;7:1129-1159. 22. Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage 2004;22:1214-1222. 23. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 2001;14:140-151. 24. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012;22:158-165. 25. Lu HB, Zuo YT, Gu H, et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA 2007;104:18265-18269. 26. Faghiri A, Stephen JM, Wang YP, Wilson TW, Calhoun VD. Changing brain connectivity dynamics: From early childhood to adulthood. Hum Brain Mapp 2018;39:1108-1117. 27. Li C, Fronczek-Poncelet J, Lange D, et al. Impact of acute sleep deprivation on dynamic functional connectivity states. Hum Brain Mapp 2019 Nov 4. 28. Li Y, Zhu Y, Nguchu BA, et al. Dynamic Functional Connectivity Reveals Abnormal Variability and Hyper-connected Pattern in Autism Spectrum Disorder. Autism Res 2019 Oct 15. 29. Yuan Y, Zhang L, Li L, et al. Distinct dynamic functional connectivity patterns of pain and touch thresholds: A resting-state fMRI study. Behav Brain Res 2019;375:112142. 30. Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 2008;9:432-441. 31. Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain 2014;137:2731-2742. 32. Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE. Levodopa-associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976-1990. Arch Neurol 2006;63:205-209. 33. Rascol O, Sabatini U, Brefel C, et al. Cortical motor overactivation in parkinsonian patients with L-dopa-induced peak-dose dyskinesia. Brain 1998:527-533. 34. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 2000;23:S8-19. 35. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448-458. 36. Wang L, Wang M, Si Q, et al. Altered brain structural topological properties in Parkinson’s disease with levodopa-induced dyskinesias. Parkinsonism Relat Disord 2019;67:36-41. 37. Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage 2007;37:343-360. 38. Aron AR,Obeso J. Is executive control used to compensate for involuntary movements in levodopa-induced dyskinesia? Mov Disord 2012;27:339-340. 39. Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord 2003;18:231-240. 40. Alam M, Rumpel R, Jin X, et al. Altered somatosensory cortex neuronal activity in a rat model of Parkinson’s disease and levodopa-induced dyskinesias. Exp Neurol 2017;294:19-31. 41. Glickstein M. How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends Neurosci 2000;23:613-617. 42. Inzelberg R, Schechtman E, Hocherman S. Visuo-motor coordination deficits and motor impairments in Parkinson’s disease. PLoS One 2008;3:e3663. 43. Christopher L, Duff-Canning S, Koshimori Y, et al. Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann Neurol 2015;77:269-280. 44. Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci 2005;8:1298-1300. 45. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 2004;306:443-447. 46. Swann NC, Cai W, Conner CR, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage 2012;59:2860-2870. 47. Laufs H, Krakow K, Sterzer P, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 2003;100:11053-11058. 48. Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 2007;104:13170-13175. 49.
Alonso-Frech F, Zamarbide I, Alegre M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 2006;129:1748-1757. 50. Marusak HA, Calhoun VD, Brown S, et al. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp 2017;38:97-108. 51. Viviano RP, Raz N, Yuan P, Damoiseaux JS. Associations between dynamic functional connectivity and age, metabolic risk, and cognitive performance. Neurobiol Aging 2017;59:135-143.
To cite this abstract in AMA style:
Q. Si, Y. Yuan, C. Gan, M. Wang, L. Wang, K. Ma, K. Zhang. Abnormal network interconnections dynamics correlate with levodopa-induced dyskinesia in Parkinson’s disease [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/abnormal-network-interconnections-dynamics-correlate-with-levodopa-induced-dyskinesia-in-parkinsons-disease/. Accessed December 27, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/abnormal-network-interconnections-dynamics-correlate-with-levodopa-induced-dyskinesia-in-parkinsons-disease/