Category: Parkinson's Disease: Genetics
Objective: The aim was to investigate the association between ZNF184 and symptoms of Parkinson’s disease (PD) in southern Chinese.
Background: Parkinson’s disease (PD) is a common movement disorder characterized by bradykinesia, rigidity and resting tremor [1]. The pathological features of PD are the abnormal aggregation of α-synuclein and the loss of dopaminergic neurons in substantia nigra [2]. Several genes have been found and proven related with PD, such as LRRK2, SNCA etc. [3,4]. The expression of those gene could influence the phenotype of PD [5]. For example, LRRK2 G2019S is associated with neuropsychiatric, dysautonomic and sleep disturbances in PD patients [6].
Recently, gene ZNF184 was found related to PD [7,8]. ZNF184 rs9468199 was associated with PD risk, especially with early onset PD in southern Chinese, indicating the involvement of gene ZNF184 in the pathogenesis of PD [8]. ZNF184 is a Krueppel-related zinc finger gene[9]. It has 19 highly conserved zinc finger motifs at the C-terminus. However, the function of gene ZNF184 to PD is unknown. Besides, as a newly-found gene related with PD, it is obliged to investigate the association between gene ZNF184 and clinical symptoms of PD, which could indicate the pathogenesis of PD and guide in clinical research.
In this study, we try to discover the clinical hallmarks of ZNF184 rs9468199 in southern Chinese PD patients.
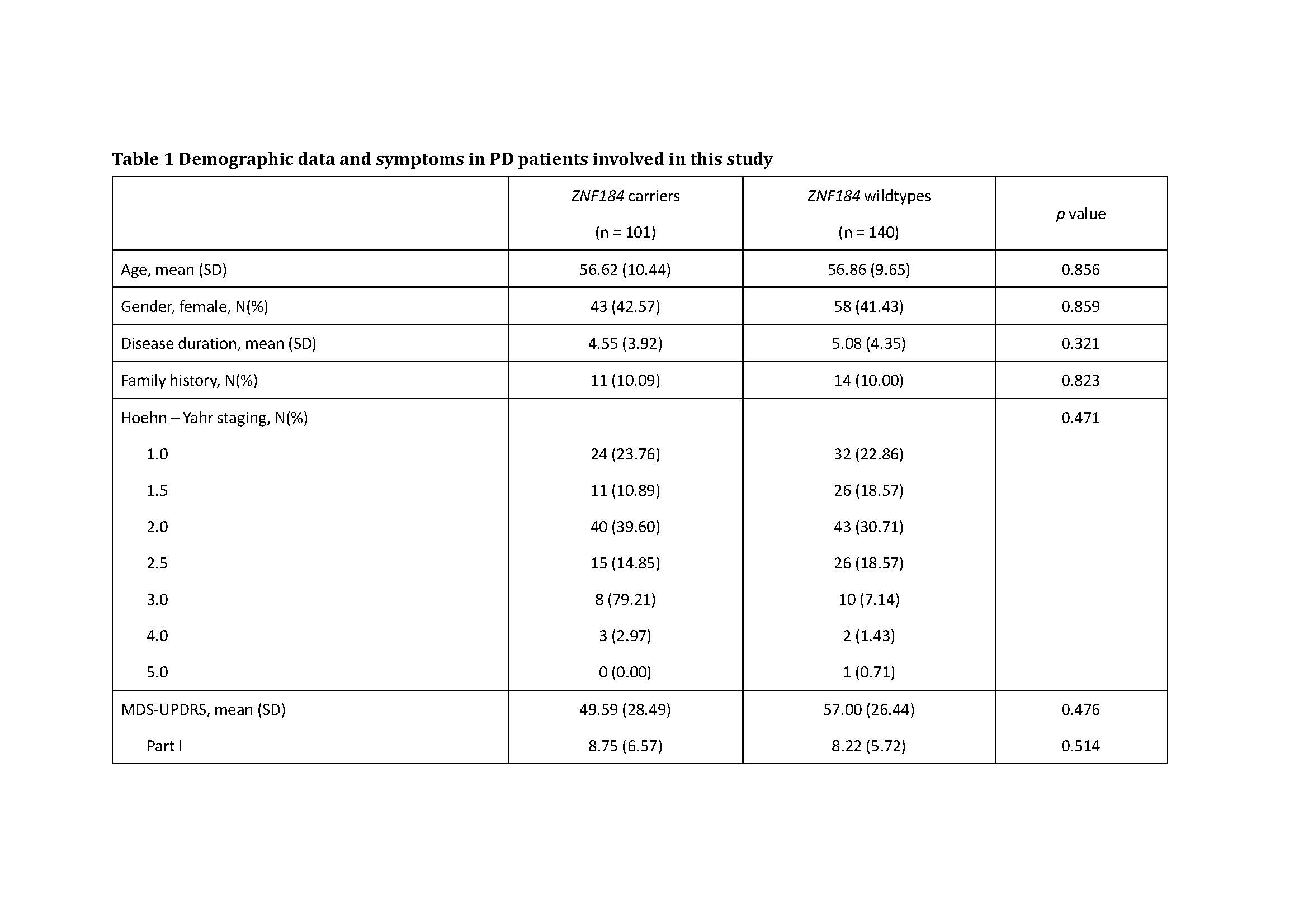
Method: 241 PD patients were recruited in this study. All patients were evaluated by Sniffin’ Sticks 16 (SS-16), Hamilton anxiety rating scale and Hamilton depression rating scale, 39-item Parkinson’s disease Questionnaire (PDQ-39) and MDS – Unified PD Rating Scale (MDS-UPDRS). Symptoms were also recorded.
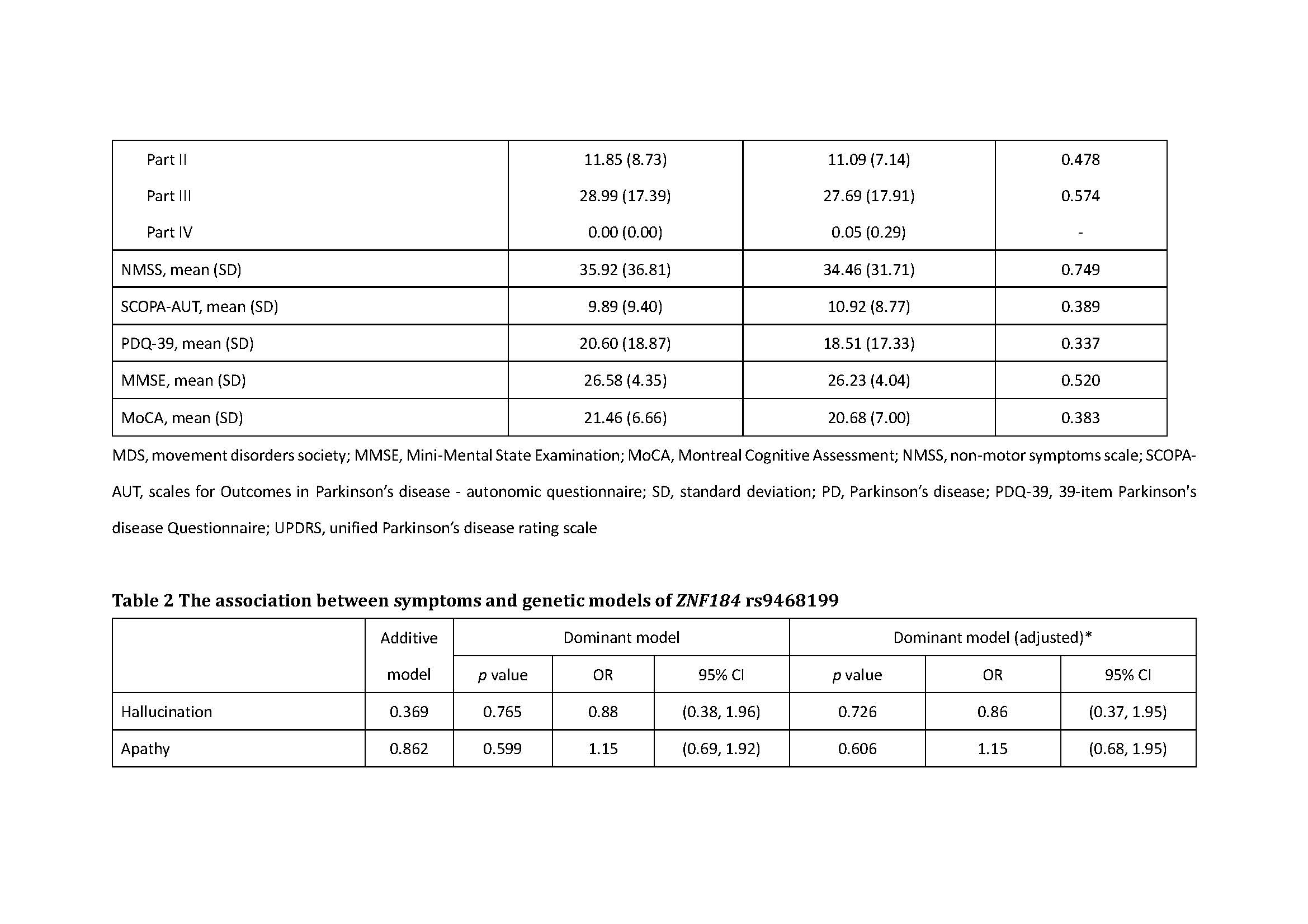
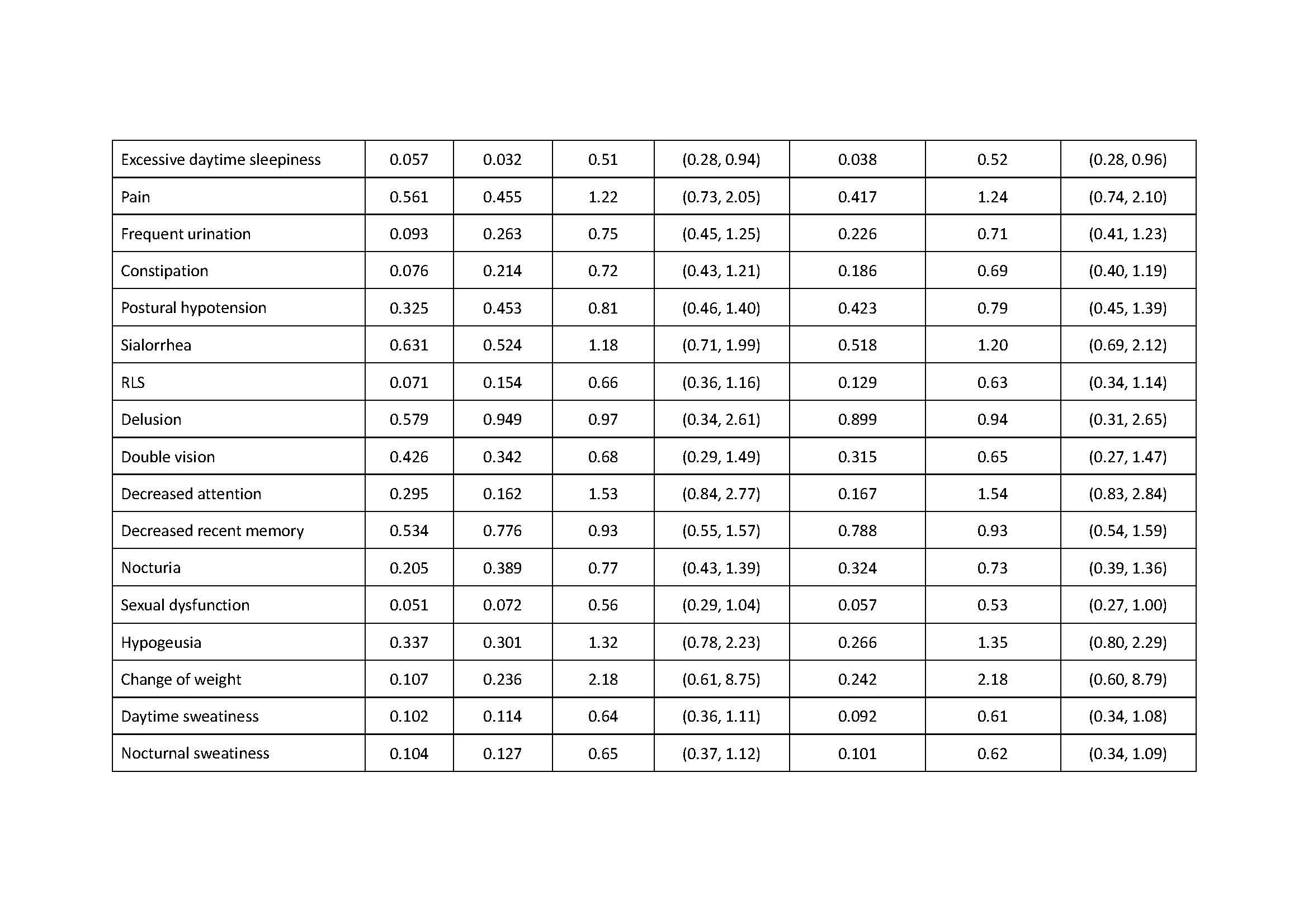
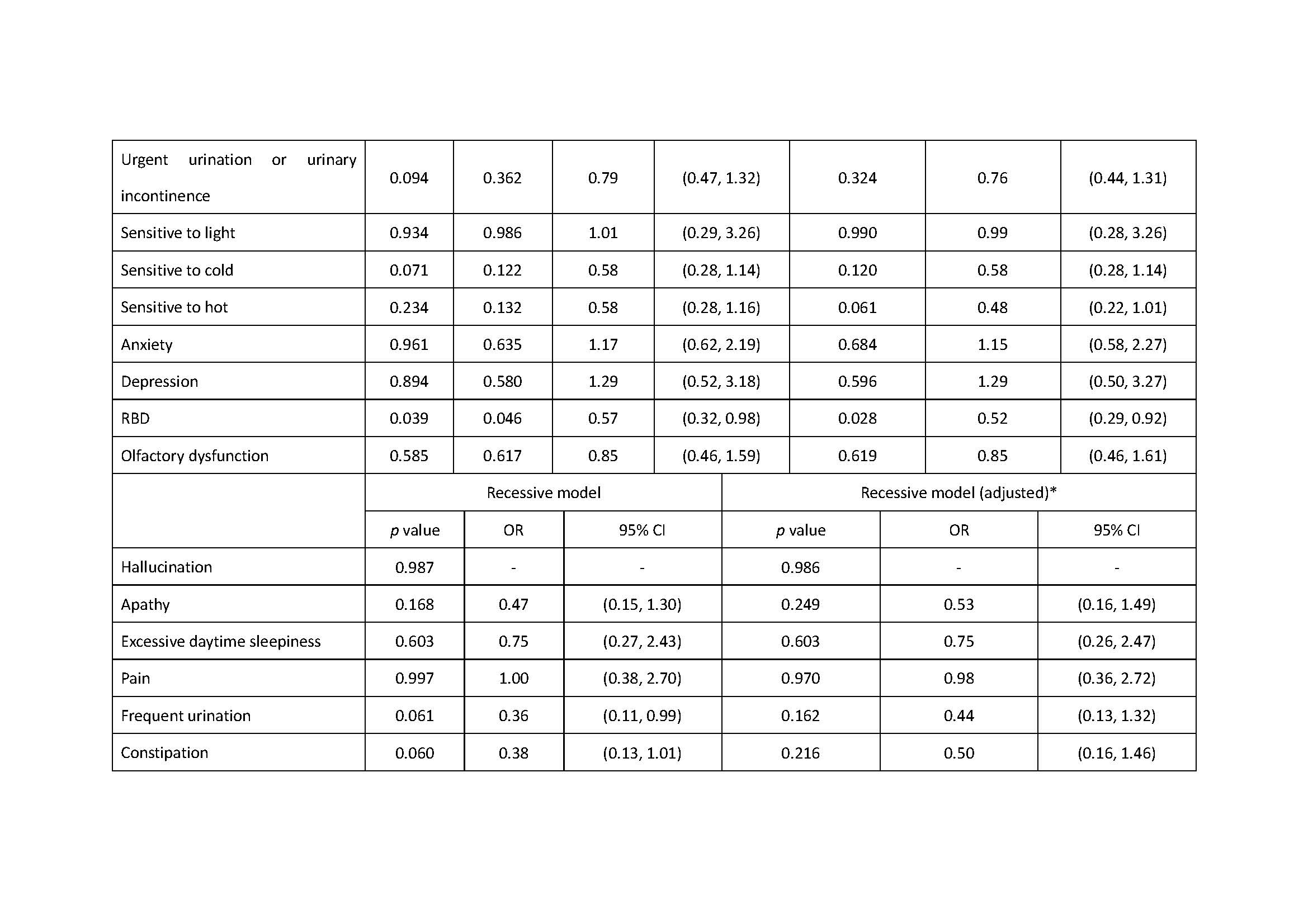
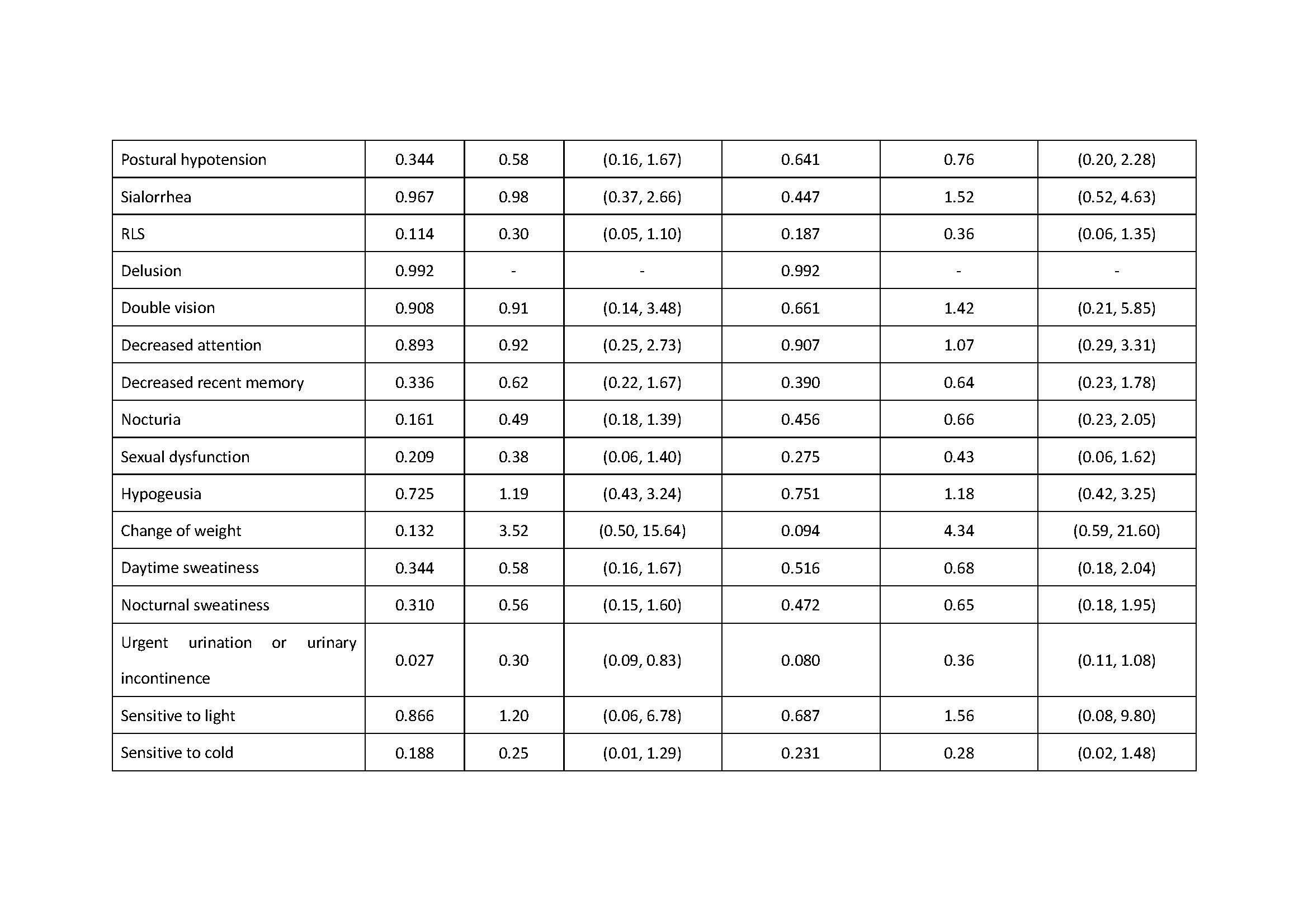
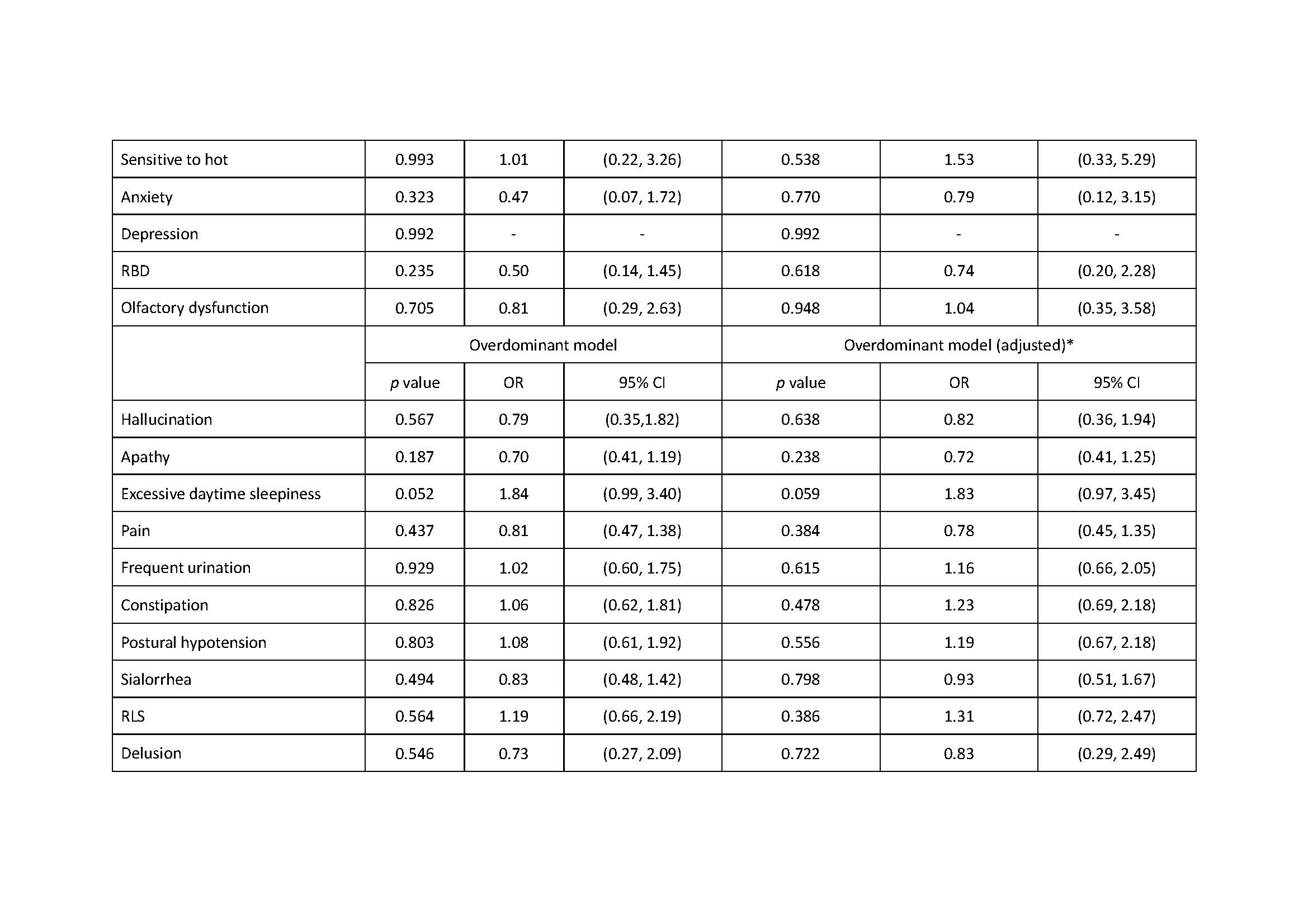
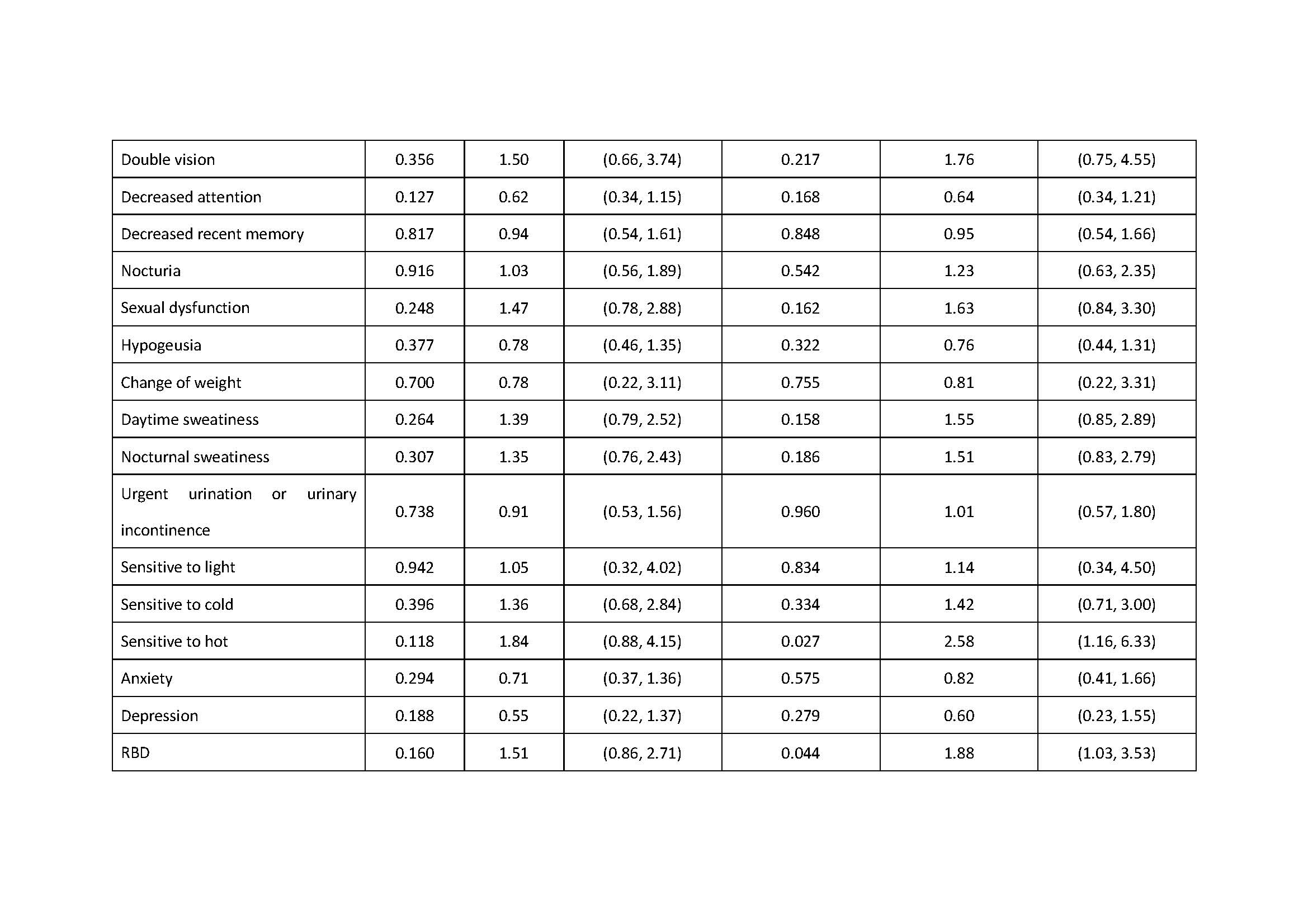
Results: There was association of RBD under additive, dominant and overdominant model (p: 0.039, additive; p: 0.028, dominant; p: 0.044, overdominant). We also found the association of excessive daytime sleepiness under the dominant model, the association of urgent urination or urinary incontinence under the recessive model and the association of sensitive to hot under the overdominant model (Excessive daytime sleepiness: p: 0.032, dominant; p: 0.038, dominant; Urgent urination or urgent incontinence: p: 0.027, recessive; Sensitive to hot: p: 0.027, overdominant).
Conclusion: ZNF184 rs9468199 was associated with the presence of RBD, excessive daytime sleepiness, urgent urination or urgent incontinence, and sensitive to hot.
References: 1. Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet (London, England) 386 (9996):896-912. doi:10.1016/s0140-6736(14)61393-3 2. Bene R, Antic S, Budisic M, Lisak M, Trkanjec Z, Demarin V, Podobnik-Sarkanji S (2009) Parkinson’s disease. Acta clinica Croatica 48 (3):377-380 3. Blanca Ramirez M, Madero-Perez J, Rivero-Rios P, Martinez-Salvador M, Lara Ordonez AJ, Fernandez B, Fdez E, Hilfiker S (2017) LRRK2 and Parkinson’s Disease: From Lack of Structure to Gain of Function. Current protein & peptide science 18 (7):677-686. doi:10.2174/1389203717666160311121748 4. Siddiqui IJ, Pervaiz N, Abbasi AA (2016) The Parkinson Disease gene SNCA: Evolutionary and structural insights with pathological implication. Scientific reports 6:24475. doi:10.1038/srep24475 5. Koros C, Simitsi A, Stefanis L (2017) Genetics of Parkinson’s Disease: Genotype-Phenotype Correlations. International review of neurobiology 132:197-231. doi:10.1016/bs.irn.2017.01.009 6. Gaig C, Vilas D, Infante J, Sierra M, Garcia-Gorostiaga I, Buongiorno M, Ezquerra M, Marti MJ, Valldeoriola F, Aguilar M, Calopa M, Hernandez-Vara J, Tolosa E (2014) Nonmotor symptoms in LRRK2 G2019S associated Parkinson’s disease. PloS one 9 (10):e108982. doi:10.1371/journal.pone.0108982 7. Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nature genetics 49 (10):1511-1516. doi:10.1038/ng.3955 8. Li G, Cui S, Du J, Liu J, Zhang P, Fu Y, He Y, Zhou H, Ma J, Chen S (2018) Association of GALC, ZNF184, IL1R2 and ELOVL7 With Parkinson’s Disease in Southern Chinese. Frontiers in aging neuroscience 10:402. doi:10.3389/fnagi.2018.00402 9. Goldwurm S, Menzies ML, Banyer JL, Powell LW, Jazwinska EC (1997) Identification of a novel Krueppel-related zinc finger gene (ZNF184) mapping to 6p21.3. Genomics 40 (3):486-489. doi:10.1006/geno.1996.4583 10. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 30 (12):1591-1601. doi:10.1002/mds.26424 11. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society 23 (15):2129-2170. doi:10.1002/mds.22340 12. Chen W, Chen S, Kang WY, Li B, Xu ZM, Xiao Q, Liu J, Wang Y, Wang G, Chen SD (2012) Application of odor identification test in Parkinson’s disease in China: a matched case-control study. Journal of the neurological sciences 316 (1-2):47-50. doi:10.1016/j.jns.2012.01.033 13. Shen SS, Shen Y, Xiong KP, Chen J, Mao CJ, Huang JY, Li J, Han F, Liu CF (2014) Validation study of REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) in east China. Sleep medicine 15 (8):952-958. doi:10.1016/j.sleep.2014.03.020 14. Goldwurm S, Menzies ML, Banyer JL, Powell LW, Jazwinska EC (1997) Identification of a Novel Krueppel-Related Zinc Finger Gene (ZNF184) Mapping to 6p21.3. Genomics 40 (3):486-489. doi:https://doi.org/10.1006/geno.1996.4583 15. Mandaviya PR, Joehanes R, Aissi D, Kuhnel B, Marioni RE, Truong V, Stolk L, Beekman M, Bonder MJ, Franke L, Gieger C, Huan T, Ikram MA, Kunze S, Liang L, Lindemans J, Liu C, McRae AF, Mendelson MM, Muller-Nurasyid M, Peters A, Slagboom PE, Starr JM, Tregouet DA, Uitterlinden AG, van Greevenbroek MMJ, van Heemst D, van Iterson M, Wells PS, Yao C, Deary IJ, Gagnon F, Heijmans BT, Levy D, Morange PE, Waldenberger M, Heil SG, van Meurs JBJ (2017) Genetically defined elevated homocysteine levels do not result in widespread changes of DNA methylation in leukocytes. PloS one 12 (10):e0182472. doi:10.1371/journal.pone.0182472 16. Garcia S, Cano-Martinez LJ, Coral-Vazquez RM, Coronel-Perez A, Gomez-Diaz B, Toledo-Lozano CG, Gallegos-Arreola MP, Davila-Maldonado L, Jimenez-Hernandez DA, Alcaraz-Estrada SL, Lopez-Hernandez LB (2017) Analysis of the rs13306560 functional variant in the promoter region of the MTHFR gene in sporadic Parkinson s disease. Neuro endocrinology letters 38 (4):257-260 17. Liao Q, Li NN, Mao XY, Chang XL, Zhao DM, Zhang JH, Yu WJ, Tan EK, Peng R (2014) MTHFR C677T variant reduces risk of sporadic Parkinson’s disease in ethnic Chinese. Acta neurologica Scandinavica 130 (1):e30-34. doi:10.1111/ane.12245 18. Liu L, Zhang L, Guo L, Yu Q, Li H, Teng J, Xie A (2018) MTHFR C677T and A1298C polymorphisms may contribute to the risk of Parkinson’s disease: A meta-analysis of 19 studies. Neuroscience letters 662:339-345. doi:10.1016/j.neulet.2017.10.060 19. Vallelunga A, Pegoraro V, Pilleri M, Biundo R, De Iuliis A, Marchetti M, Facchini S, Formento Dojot P, Antonini A (2014) The MTHFR C677T polymorphism modifies age at onset in Parkinson’s disease. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 35 (1):73-77. doi:10.1007/s10072-013-1545-z 20. Yuan L, Song Z, Deng X, Xiong W, Yang Z, Deng H (2016) Association of the MTHFR rs1801131 and rs1801133 variants in sporadic Parkinson’s disease patients. Neuroscience letters 616:26-31. doi:10.1016/j.neulet.2016.01.031 21. Zahra C, Tabone C, Camilleri G, Felice AE, Farrugia R, Bezzina Wettinger S (2016) Genetic causes of Parkinson’s disease in the Maltese: a study of selected mutations in LRRK2, MTHFR, QDPR and SPR. BMC medical genetics 17 (1):65. doi:10.1186/s12881-016-0327-x 22. Hashida H, Goto J, Zhao N, Takahashi N, Hirai M, Kanazawa I, Sakaki Y (1998) Cloning and mapping of ZNF231, a novel brain-specific gene encoding neuronal double zinc finger protein whose expression is enhanced in a neurodegenerative disorder, multiple system atrophy (MSA). Genomics 54 (1):50-58. doi:10.1006/geno.1998.5516
To cite this abstract in AMA style:
B.Z Zhang, G.L Li. Association between ZNF184 and symptoms of Parkinson’s disease in southern Chinese [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/association-between-znf184-and-symptoms-of-parkinsons-disease-in-southern-chinese/. Accessed October 21, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/association-between-znf184-and-symptoms-of-parkinsons-disease-in-southern-chinese/