Session Information
Date: Thursday, June 23, 2016
Session Title: Dystonia
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To compare the effects of botulinum toxin type B (BtB) versus placebo in cervical dystonia (CD).
Background: This is an update of a Cochrane review. CD is a frequent and disabling disorder characterized by involuntary head posturing. Botulinum toxin type A (BtA) is usually the first line therapy although BtB is also an approved indication.
Methods: Search Methods: Cochrane Movement Disorders Group trials register, CENTRAL, MEDLINE, EMBASE, as well as reference lists of articles and conference proceedings, last run in December 2015. Selection criteria: double-blind, parallel, randomized, placebo-controlled trials of BtB in adult patients with CD. Data collection: two independent authors assessed records, selected included studies, extracted data and evaluated the risk of bias. Disagreements were solved by consensus or by a third element.
Results: We included 4 studies (n=441) with an overall moderate quality. Most studies excluded patients known to have poorer response to Bt treatment (e.g. anterocollis). None of the studies were independently funded. All evaluated the effect of a single treatment session. BtB was associated with an improvement of 15% in the patient’s baseline clinical status as assessed by investigators, reducing 7 points in TWSTRS-total score at week 4 after injection (95% CI: 4.54 to 9.01) (Figure 1). Mean difference in TWSTRS-pain score at week 4 was 2.41 (95% CI: 0.82 to 4.01). Subjective clinician and patient assessments of change favoured BtB over placebo. Neither withdrawals due to adverse events nor the proportion of patients with adverse events were significantly different between groups. However, BtB-treated patients had a 7.65 (95% CI: 2.75 to 21.32) and a 6.78 (95% CI: 2.42 to 19.05) increased risk of dry mouth and dysphagia, respectively. 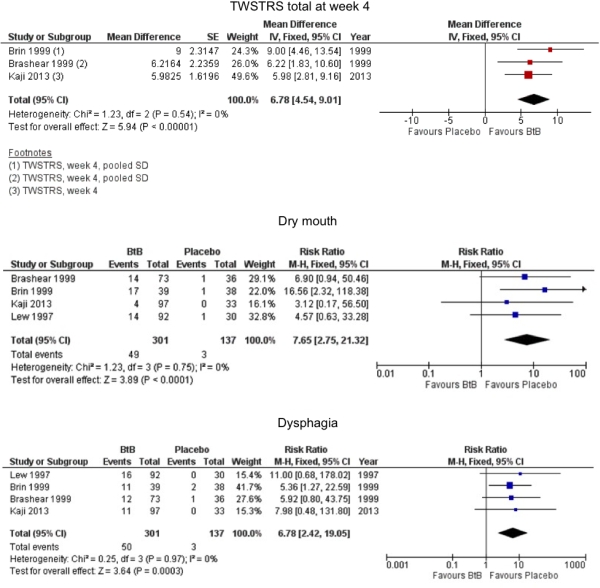 Statistical heterogeneity between study results was low for most outcomes. Subgroup analysis dose failed to show significant efficacy or safety differences between doses.
Statistical heterogeneity between study results was low for most outcomes. Subgroup analysis dose failed to show significant efficacy or safety differences between doses.
Conclusions: A single BtB-treatment session is efficacious in improving CD, although patients are at an increased risk of dry mouth and dysphagia.  There is no random controlled data evaluating the effectiveness and safety (including immunogenicity) of repeated injection cycles. Data is also lacking to conclude on the optimal treatment intervals and doses, usefulness of guidance techniques for injection, and impact on quality of life.
There is no random controlled data evaluating the effectiveness and safety (including immunogenicity) of repeated injection cycles. Data is also lacking to conclude on the optimal treatment intervals and doses, usefulness of guidance techniques for injection, and impact on quality of life.
To cite this abstract in AMA style:
R.E. Marques, G.S. Duarte, F.B. Rodrigues, M. Castelão, J.J. Ferreira, P. Moore, J. Costa. Botulinum toxin type B therapy for cervical dystonia – 2015 update of a Cochrane systematic review and meta-analysis [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/botulinum-toxin-type-b-therapy-for-cervical-dystonia-2015-update-of-a-cochrane-systematic-review-and-meta-analysis/. Accessed December 26, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/botulinum-toxin-type-b-therapy-for-cervical-dystonia-2015-update-of-a-cochrane-systematic-review-and-meta-analysis/
