Category: Dystonia: Clinical Trials and Therapy
Objective: To evaluate the effect of abobotulinumtoxinA (aboBoNT-A) on pain associated with cervical dystonia (CD) in routine clinical practice.
Background: Pain is considered as the most frequent and challenging symptom of CD. Patient surveys have found that the presence of pain is the main reason that patients seek treatment.1, 2
Method: This meta-analysis was based on first treatment cycle data from 3 observational studies (INTEREST IN CD1, INTEREST IN CD2 and ANCHOR-CD). Due to differences in methodology, some variables were not consistently collected across the component studies. Only data from patients treated with aboBoNT-A and with data collected both at baseline and Visit 2 from neurology clinics were considered. Changes in Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) scores were compared versus baseline using a two-sided paired t-test at the 5% significance level.
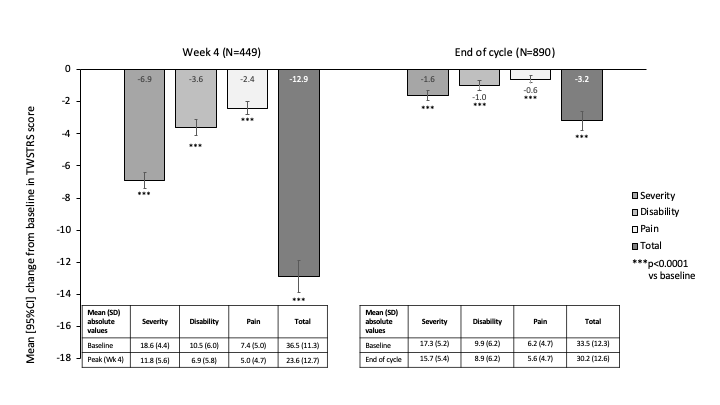
Results: The initial meta-analysis included data from 1091 CD patients treated with aboBoNT-A at 181 neurology centers in 35 countries. AboBoNT-A median [range] dose was 500U [50–1700] with a mean injection cycle duration of 16 weeks (median [range]: 99 [43–582] days). Post-hoc analysis on TWSTRS scores were based on 920 patients with available data. In this population, TWSTRS scores significantly improved from baseline to Week 4 and did not return fully to baseline at end of the injection cycle [Figure]. TWSTRS Total scores significantly reduced by a mean [95%CI] of -12.9 [-13.9, -11.8] points at Week 4 (N=449) and by -3.2 [-3.8, -2.7] points at the end of injection cycle (N=890). All TWSTRS domains (severity, disability and pain) contributed to the overall improvement, with significant reductions versus baseline both at peak effect (Week 4) and at end of injection cycle. Pain was significantly reduced at Week 4 by a mean [95%CI] of -2.4 [-2.8, -2.0] (p<0.0001), which equated to >30% improvement from baseline.
Conclusion: These analyses show that treatment with aboBoNT-A in routine clinical practice led to a significant reduction in pain associated with CD. The magnitude of improvement is consistent with aboBoNT-A phase 3 study results reported by Truong in 2005.3
References: 1. Novak I, Campbell L, Boyce M, Fung VS. Botulinum toxin assessment, intervention and aftercare for cervical dystonia and other causes of hypertonia of the neck: international consensus statement. Eur J Neurol. 2010;17 Suppl 2:94-108. 2. Camargo CH, Cattai L, Teive HA. Pain Relief in Cervical Dystonia with Botulinum Toxin Treatment. Toxins (Basel). 2015;7(6):2321-35. 3. Truong D, Duane DD, Jankovic J, Singer C, Seeberger LC, Comella CL, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20(7):783-91.
To cite this abstract in AMA style:
R. Trosch, V. Misra, P. Maisonobe, S. Om. The effect of botulinum toxin A on pain associated with cervical dystonia (CD) in routine clinical practice [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/the-effect-of-botulinum-toxin-a-on-pain-associated-with-cervical-dystonia-cd-in-routine-clinical-practice/. Accessed January 7, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-effect-of-botulinum-toxin-a-on-pain-associated-with-cervical-dystonia-cd-in-routine-clinical-practice/