Session Information
Date: Wednesday, September 25, 2019
Session Title: Epidemiology
Session Time: 1:15pm-2:45pm
Location: Les Muses, Level 3
Objective: To establish a registry of PD patients in Thailand treated with subcutaneous apomorphine (APO) injection or infusion to allow prospective data collection from the start of therapy and subsequent evaluation of treatment efficacy, patient outcomes, and cost-effectiveness. Here we report data for the first 40 patients undergoing APO infusion treatment.
Background: Subcutaneous APO is a well-established and effective PD therapy. APO infusion became available in Thailand in 2013 and APO injection in 2017. The registry was launched at the initiation of APO therapy in Thailand in 2015.
Method: All patients treated with APO infusion at the Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders, the only dedicated centre for PD in Thailand, are enrolled in the registry and followed-up at regular clinic visits. The multidisciplinary team involved in patient care collect and upload data to the registry for treatment monitoring and analysis using electronic datasheets.
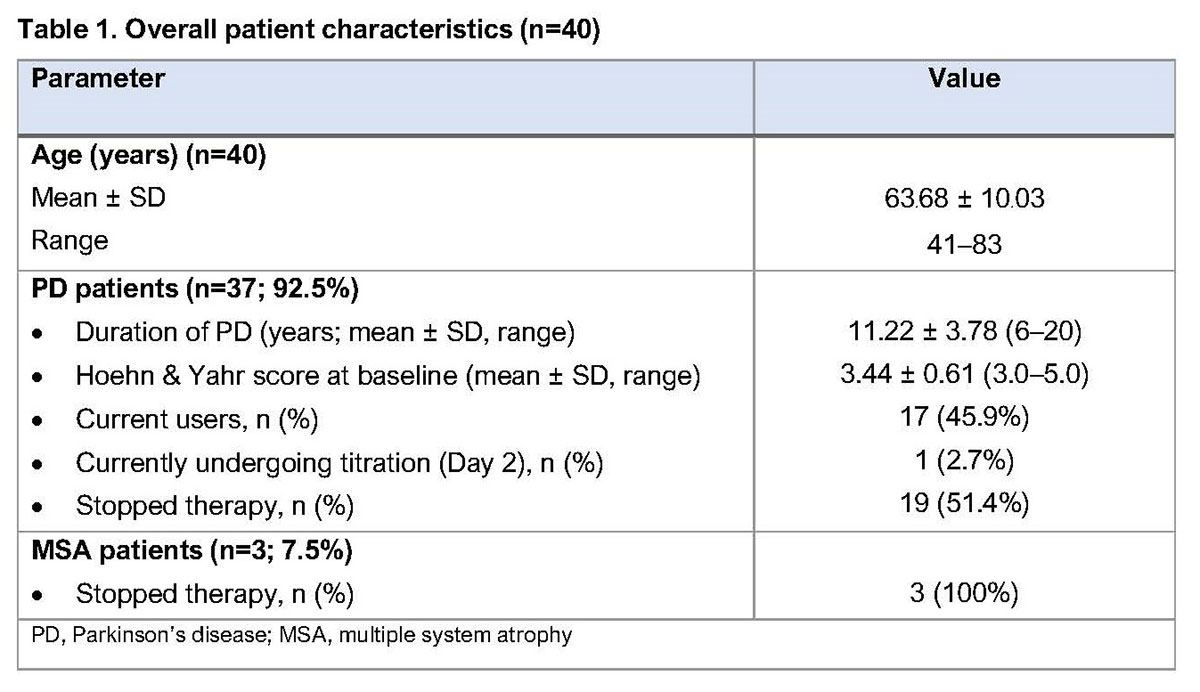
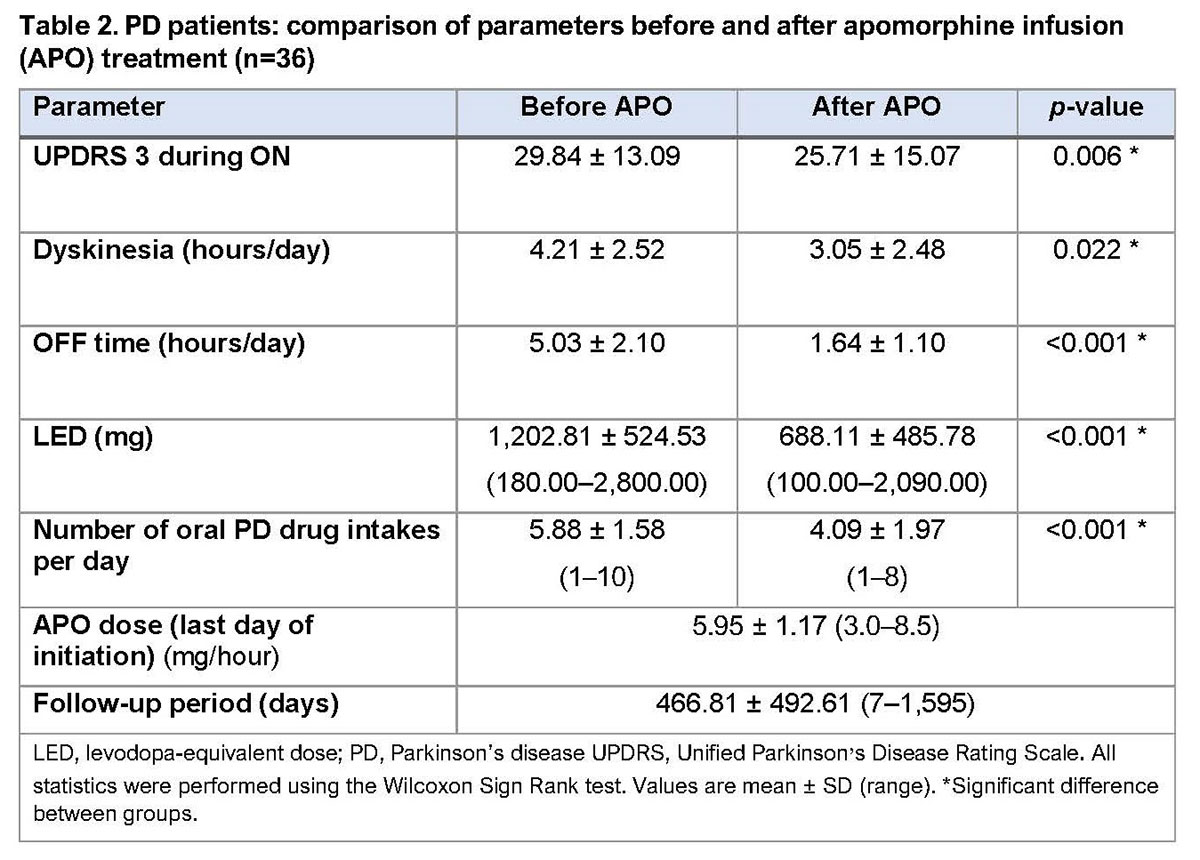
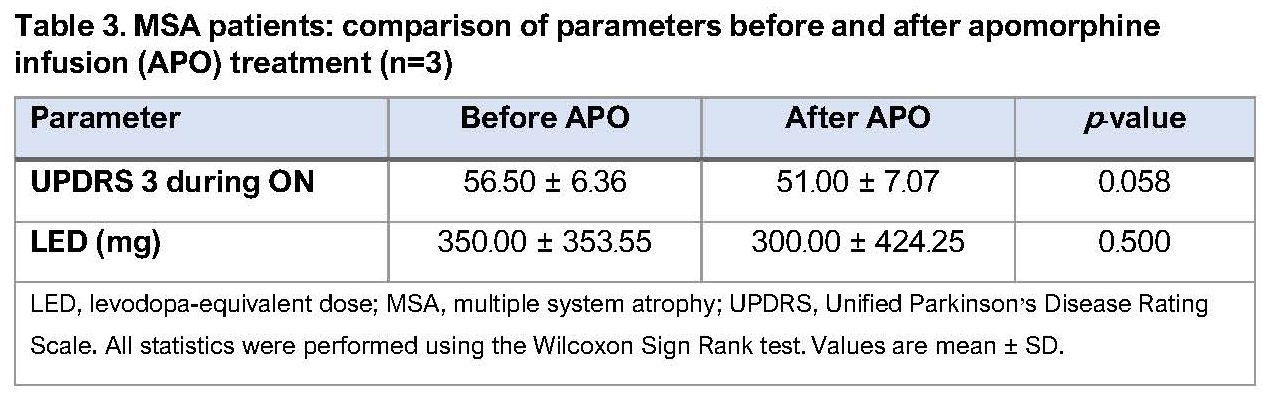
Results: To date, 40 patients (37 PD; 3 multiple system atrophy [MSA]; Table 1) have undergone APO infusion treatment (1 undergoing initiation at the time of the analysis). Following initiation, PD patients were discharged on a mean (± SD) dose of 5.95 ± 1.17 mg/hour. The mean (± SD) follow-up period is 466.81 ± 492.61 days. PD patients (n=36) show significant improvements in UPDRS score, daily OFF time and dyskinesia, and reductions in levodopa-equivalent dose and number of daily intakes of oral PD medication versus pre-treatment values (Table 2). Around 50% of PD patients have discontinued therapy during the follow-up period. Further outcomes data, including nocturnal parameters, gait parameters and bradykinesia index, have been collected and are currently being analysed. MSA patients showed no significant benefits of treatment and all ceased therapy (Table 3).
Conclusion: The Thai apomorphine treatment registry will allow standardised, prospective collection of patient data and continuous follow-up from the time of initiation. Analysis of data from this comprehensive database will have benefits for patient care, inform healthcare policy and provision, and provide a resource for future research.
To cite this abstract in AMA style:
R. Bhidayasiri, O. Phokaewvarangkul, K. Boonpang, T. Boonmongkol, Y. Thongchuam, N. Kantachadvanich, J. Sringean, P. Panyakaew, P. Jagota. Establishing the first apomorphine treatment registry in Thailand: prospective data collection to inform optimal care of Parkinson’s disease patients [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/establishing-the-first-apomorphine-treatment-registry-in-thailand-prospective-data-collection-to-inform-optimal-care-of-parkinsons-disease-patients/. Accessed October 22, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/establishing-the-first-apomorphine-treatment-registry-in-thailand-prospective-data-collection-to-inform-optimal-care-of-parkinsons-disease-patients/