Session Information
Date: Tuesday, September 24, 2019
Session Title: Education / History in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Agora 2 West, Level 2
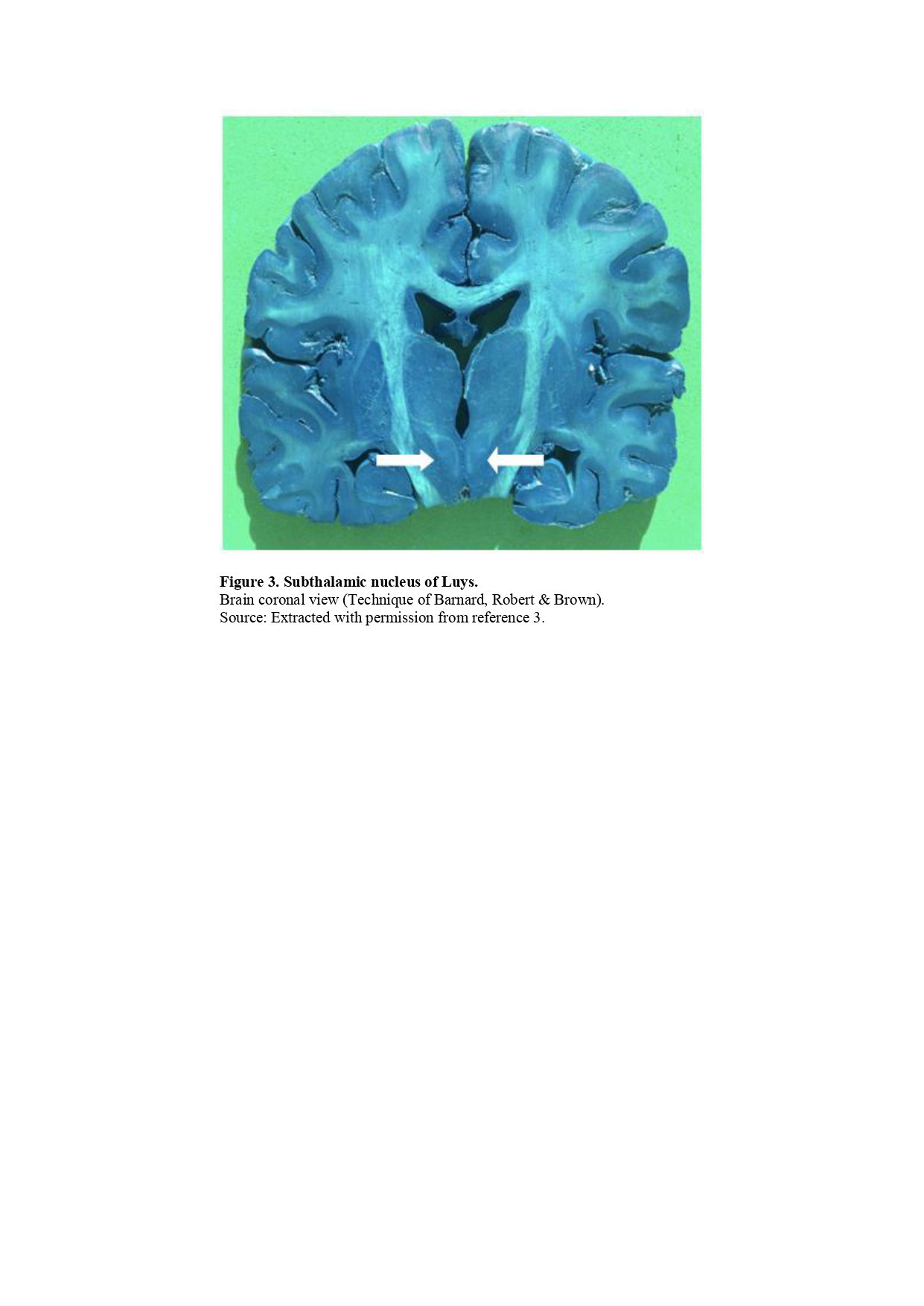
Objective: The review provides detailed material about the Subthalamic Nucleus of Luys, including pathophisiolgy, hystorical notes about Luys, embryology, anatomy, principal afferents and efferents pathways, as well as, clinical correlations and surgery treatments involving the STN.
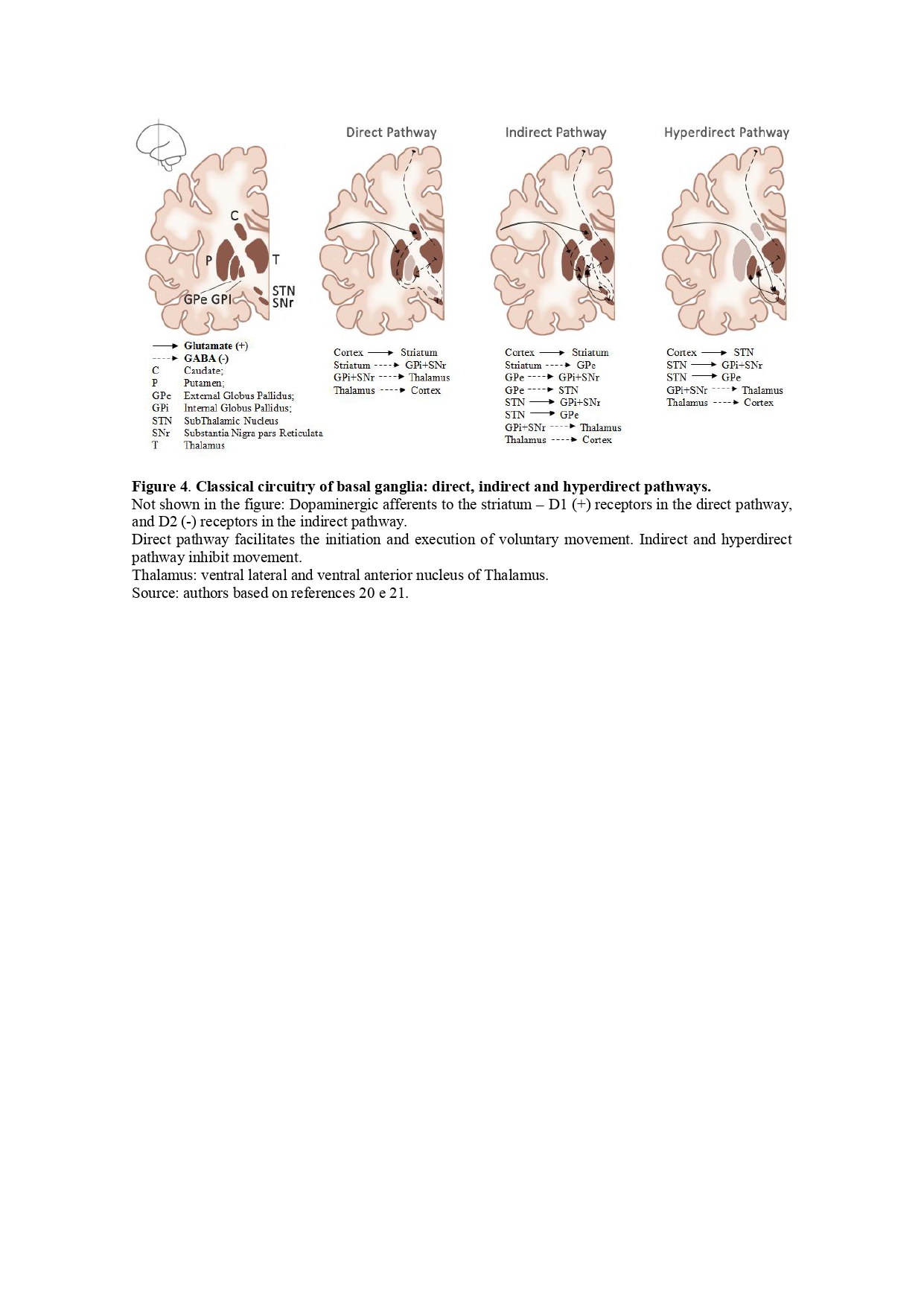
Background: In 1912, Samuel Wilson crafted the term “extrapyramidal” with the purpose of characterizing a set of anatomical structures represented by the basal ganglia which are involved in motor control and when dysfunctional, result in the occurrence of movement disorders.The subthalamic nucleus (STN) represents a critical component of the basal ganglia with a pivotal function in the modulation of movement.
Method: The authors review the subthalamic nucleus (STN) since its description up to current concepts of physiology, clinical and therapeutic relevance. The role of Jules Bernard Luys in the description of the STN is emphasized, as well as the anatomy, physiology, the importance of the STN in the genesis of movement disorders, and its’ potential role as a target for neuromodulation.
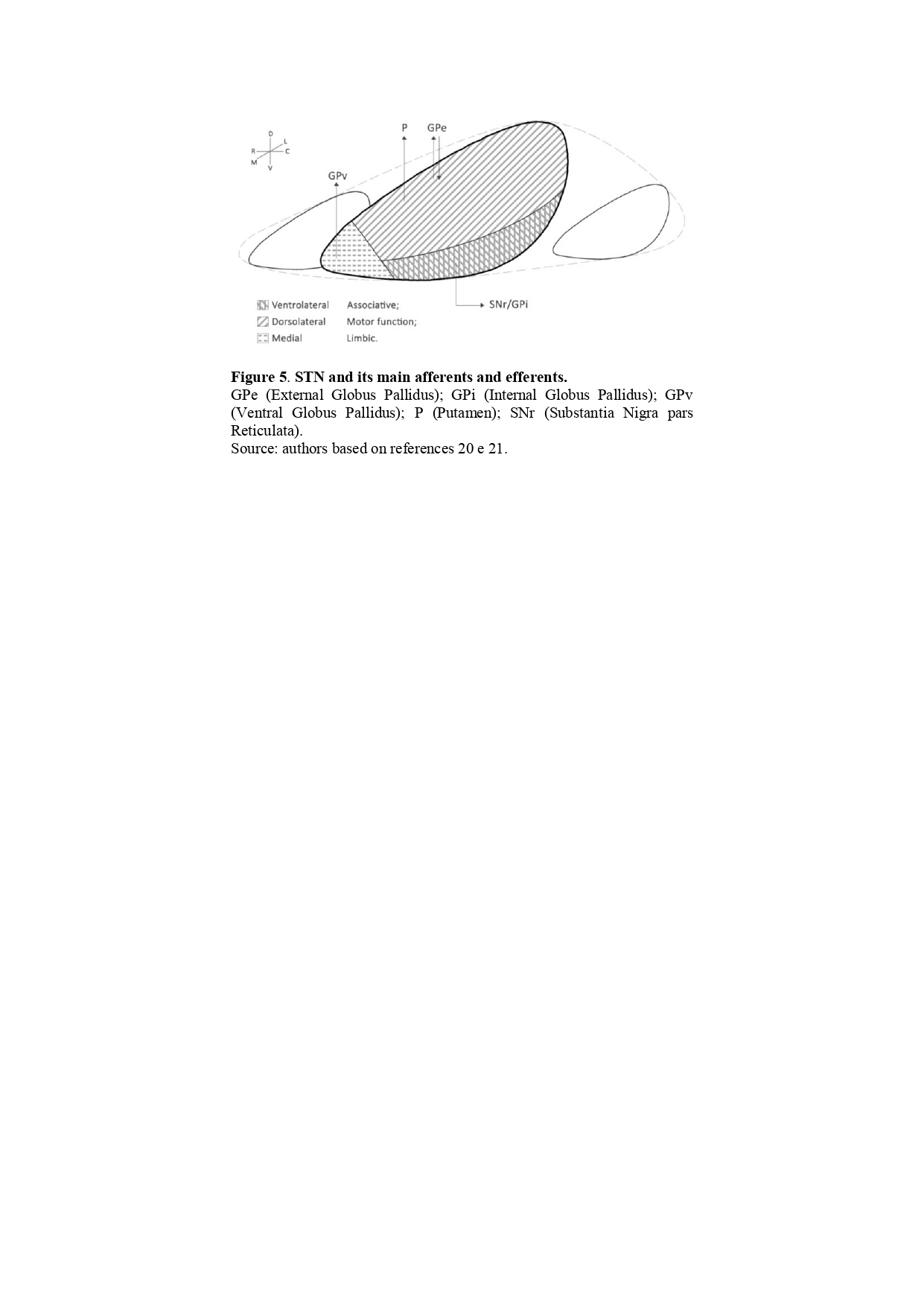
Results: Luys contributions to neuroanatomy were decisive, including the identification of anatomical structures including the identification of a structure in the forebrain that is still attached to his name: subthalamic nucleus, or corpus Luysii. Beyond the classical cortico-basal ganglia-thalamo-cortical circuitry, the STN connects also to the brainstem. It can be divided into three functional subregions: limbic, motor and associative. Unilateral lesion in STN leads to contralateral hemiballismus, a classic clinical observation. Non-motor manifestations are less frequent but may include neuropsychiatric disorders such as hyperphagia, aggressiveness, anxiety, irritability, euphoria, and pleomorphic impulse control disorders.
Conclusion: The STN is a small nucleus that functions as a relay of motor and non-motor circuits important for human functionality. The relationships of NST with movement disorders, in particular hemibalism and PD are well known. The neuromodulation of STN has been widely used in the last decades, however with the refinement of the understanding of its multifaceted functions, as well as the use of new technical advances that could modulate specific subregions, the STN remains as a potential target to be further explored as a therapeutic target.
References: 1. Denny-Brown D. The basal ganglia and their relation to disorders of movement. London: Oxford University Press, 1962. 2. Obeso JA, Rodriguez MC, DeLong MR. Basal ganglia pathophysiology. A critical review. In: Obeso JA, DeLong MR, Ohye C, Marsden CD (Eds). The basal ganglia and new surgical approaches for Parkinson´s disease. Advances in Neurology Volume 74. Philadelphia: Lippincot-Raven, 1997, pp.3-18. 3. Teive HAG. Núcleos da base, estruturas correlatas e vias extrapiramidais. In: Meneses MS. Neuroanatomia aplicada. Terceira edição. Rio de Janeiro: Guanabara Koogan, 2011. 4. Pearce J. The subthalamic nucleus and Jules Bernard Luys (1828-1897). J NeurolNeurosurg Psychiatry 2001; 71 (6): 783. 5. DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007; 64: 20-24. 6. Hameleers R, Temel Y, Visser-Vandewalle V. History of the Corpus Luysii: 1865-1995. Arch Neurol 2006; 63 (9): 1340-1342. 7. Temel Y, Blokland A, Steinbusch HW, Visser-Vanderwalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. ProgNeurobiol 2005; 76: 393-413. 8. Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology 2008; 70: 1991–1995. 9. Goetz CG, Bonduelle M, Gelfand T. Charcot Constructing Neurology. New York: Oxford University Press; 1995, pp. 217-267. 10. Parent M, Parent A. Jules Bernard Luys in Charcot´s penumbra. Front NeurolNeurosci. Basel, Karger, 2011, 29: 125-136. 11. Parent A. Jules Bernard Luys and the Subthalamic Nucleus. MovDisord 2002; 17(1): 181–185. 12. Parent A, Parent M, Leroux-Hugon V. Jules Bernard Luys: a singular figure of 19th century neurology. Can J NeurolSci 2002; 29(3): 282–288. 13. Lanska DJ. Chapter 33: the history of movement disorders. In: HandbClinNeurol 2010; 95: 501–46. 14. Rijcke S. Light Tries the Expert Eye: The Introduction of Photography in Nineteenth-Century Macroscopic Neuroanatomy. J HistNeurosci 2008; 17(3): 349–366. 15. Clarac F, Barbara JG, Broussolle E, Poirier J. Figures and institutions of the neurological sciences in Parisfrom 1800 to 1950. Introduction and Part I: Neuroanatomy. Rev Neurol (Paris) 2012; 168(1): 2–14. 16. Marani E, Heida T, Lakke EA, Usunoff KG. The subthalamic nucleus. Part I: development, cytology, topography and connections. AdvAnatEmbryol Cell Biol 2008; 198: 1–113, vii. 17. Hamani C, Florence G, Heinsen H, Plantinga BR, Temel Y, Uludag K, et al. Subthalamic Nucleus Deep Brain Stimulation: Basic Concepts and Novel Perspectives. eNeuro 2017; 4(5): ENEURO.0140–17.2017; DOI: 10.1523/ENEURO.0140–17.2017. 18. Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Reviews 1995; 20: 128–154. 19. Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain 2004; 127: 4–20. 20. Anteraper SA, Guell X, Whitfield-Gabrieli S, Triantafyllou C, Mattfeld AT, Gabrieli JD et al. Resting-State Functional Connectivity of the Subthalamic Nucleus to Limbic, Associative, and Motor Networks. Brain Connectivity 2018;8(1):22–32. 21. Bonnevie T, Zaghloul KA. The Subthalamic Nucleus: Unravelling New Roles and Mechanisms in the Control of Action. Neuroscientist 2018;1073858418763594. 22. Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neuroscience Research 2002;43:111–117. 23. Rossi PJ, Gunduz A, Okun MS. The Subthalamic Nucleus, Limbic Function, and Impulse Control. Neuropsychol Rev 2015; 25: 398–410. 24. Cardoso F. Chorea, Ballism, and Athetosis. In: Jankovic J, Tolosa E. Parkinson’s disease & Movement Disorders. Fifth Edition, Philadelphia: Lippincott, Williams & Wilkins, 2007: 236–245. 25. Canfield RM, Putnam JJ. A case of acute hemiplegic chorea; with autopsy and remarks. Bost Med &Surg J 1884; 111: 220–223. 26. Donaldson IM, Marsden CD, Schneider SA, Bhatia KP. Functions of the basal ganglia. In: Donaldson IM, Marsden CD, Schneider SA, Bhatia KP. Marsden´s book of movement disorders. Oxford: Oxford University Press, 2012, pp.63-136. 27. Martin JP. Hemichorea resulting from a local lesion of the brain: the syndrome of the body of Luys. Brain 1927; 50: 637–651. 28. Whittier JR, Mettler FA. Studies on the subthalamus of the rhesus monkey. I. Anatomy and fiber connections of the subthalamic nucleus of Luys. J Comp Neurol 1949; 90: 281–314. 29. Whittier JR, Mettler FA. Studies on the subthalamus of the rhesus monkey. II. Hyperkinesia and other physiologic effects of subthalamic lesions, with special reference to the subthalamic nucleus of Luys. J Comp Neurol 1949; 90: 319–372. 30. Dewey RB, Jr, Jankovic J. Hemiballism-Hemichorea. Clinical and Pharmacologic Findings in 21 Patients. Arch Neurol 1989; 46: 862–867. 31. Coral P, Teive HAG, Werneck LC. Hemibalismo. Relato de Oito casos. ArqNeuropsiquiatr 2000; 58(3-A): 698–703. 32. Choi BS, Shen G, Nan G, Kim JM, Jung KY, Jeon B. Dramatic psychiatric and behavioral symptoms following a subthalamic lesion. J ClinNeurosci 2017; doi: 10.1016/j.jocn.2017.10.051. 33. Klinger N, Mittal S. Deep brain stimulation for seizure control in drug-resistant epilepsy. Neurosurg Focus 2018; 45 (2): E4. 34. Zhou JJ, Chen T, Farber SH, Shetter AG, Ponce FA. Open-loop deep brain stimulation for the treatment of epilepsy: a systematic review of clinical outcomes over the past decade (2008–present). Neurosurg Focus 2018; 45 (2): E5. 35. Gey, Laura, Gernert, Manuela, Löscher, Wolfgang. Continuous bilateral infusion of vigabatrin into the subthalamic nucleus: Effects on seizure threshold and GABA metabolism in two rat models, Neurobiology of Disease 2016; doi: 10.1016/j.nbd.2016.03.012. 36. Sankhe M, Churi O. Newer advances in lesional surgery for movement disorders. Neurol India 2018; 66, Suppl S1:113–121. 37. Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson´s disease: A special essay on the 200th anniversary of the shaking palsy. MovDisord 2017; 32 (9): 1264-1310. 38. Lang AE, Espay AJ. Disease modification in Parkinson´s disease: Current approaches, challenges, and future considerations. MovDisord 2018; 33 (5): 660-677. 39. Braak H, Del Tredici K, Rüb U, deVos RA, Jansen Steur EN, Braak E. Staging on brain pathology related to sporadic Parkinson´s Disease.Neurobiol Aging 2003; 24 (2): 197-211. 40. Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 2012; 11: 429–442. 41. Okun MS, Gallo BV, Manndybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomized controlled trial. Lancet Neurol 2012; 11: 140–49. 42. Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008; 359(20): 2121–2134. 43. Brandão P, Grippe TC, Modesto LC, Ferreira AGF, Silva FM, Pereira FF, et al. Decisions about deep brain stimulation therapy in Parkinson’s disease. ArqNeuropsiquiatr 2018; 76 (6): 411-420. 44. Diamond A, Shahed J, Jankovic J. The effects of subthalamic nucleus deep brain stimulation on parkinsonian tremor. J NeurolSci 2007; 260 (1-2): 199–203. 45. Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995; 345: 91–95. 46. Williams NR, Foote KD, Okun MS. STN vs. GPi Deep Brain Stimulation: Translating the Rematch into Clinical Practice. MovDisordClinPract 2014; 1(1): 24–35. 47. Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, Tezenas du Moncel S. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J NeurolNeurosurg Psychiatry 2002; 72: 701–707. 48. Jahanshahi M, Obeso I, Baunez C, Alegre M, Krack P. Parkinson’s Disease, the Subthalamic Nucleus, Inhibition, and Impulsivity. MovDisord 2015; 30(2): 128–40. 49. Widge AS, Agarwal P, Giroux M, Farris S, Kimmel RJ, Hebb AO. Psychosis from subthalamic nucleus deep brain stimulator lesion effect. SurgNeurolInt 2013; 4: 7. 50. Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism RelatDisord 2016; 25: 1–9. 51. Obeso I, Wilkinson L, Casabona E, Speekenbrink M, Bringas ML, Álvarez M, et al. The subthalamic nucleus and inhibitory control: impact of subthalamotomy in Parkinson’s disease. Brain 2014:137; 1470–1480. 52. Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson´s disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry 2009; 80 (9): 979-985. 53. Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson´s Disease: initial and long-term response. Brain 2005; 128 (Pt 3): 570-583. 54. Luys J. Recherches sur le système cérébro-spinal, sa structure, ses fonctions et ses maladies. Paris, Baillière, 1865. 55. Wilson SAK. Progressive lenticular degeneratio. A familial nervous disease associated with cirrhosis of the liver. Doctoral dissertation. Brain, Oxford, 1912, 34: 295-507. 56. Carpenter MB, Whittier JR, Mettler FA: Analysis of choreoid hyperkinesia in the rhesus monkey. Surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol 1950; 92: 293–331. 57. Bergman H, Wichmann T, DeLong MR: Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 1990; 249: 1436–1438. 58. Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci 1993; 5: 382-389. 59. Trillet M, Vighetto A, Croisile B, Charles N, Aimard G. Hemiballismus with logorrhea and thymo-affective disinhibition caused by hematoma of the left subthalamic nucleus. Rev Neurol 1995; 151 (6–7): 416-419. 60. Chabardès S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL: Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord 2002; 4 (Suppl 3): S83–S93.
To cite this abstract in AMA style:
G. Froehner, A. Meira, F. Germiniani, M. Meneses, R. Munhoz, H. Teive. Subthalamic Nucleus of Luys: A review. What has evolved since 1865? [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/subthalamic-nucleus-of-luys-a-review-what-has-evolved-since-1865/. Accessed January 2, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/subthalamic-nucleus-of-luys-a-review-what-has-evolved-since-1865/