Session Information
Date: Tuesday, September 24, 2019
Session Title: Dystonia
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: Embouchure dystonia (ED) is a rare focal, task-specific dystonia affecting brass and woodwind musicians. Its underlying pathophysiology is poorly understood [1].
Background: Transcranial magnetic stimulation (TMS), functional MRI (fMRI) and magnetoencephalography (MEG) have been used to investigate more common dystonias as well as ED. Disorganization of the somatosensory homunculus, alteration in sensorimotor processing, abnormal cortical plasticity and loss of cortical inhibition have all been implicated as proposed mechanisms [2-6].
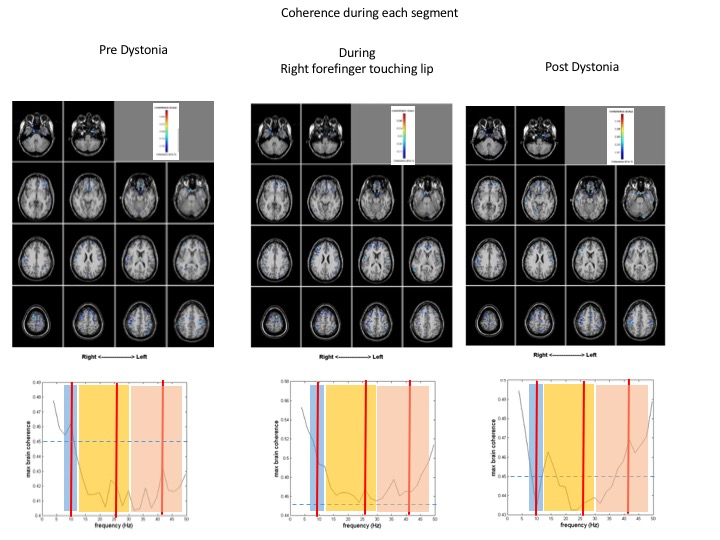
Method: MEG data was collected from a patient with ED at rest, while using tactile stimulation (index finger) to evoke dystonic activity normally triggered by playing the flute and following cessation of dystonic activity. MEG-coherence source imaging (MEG-CSI) was used to noninvasively map the connectivity between brain regions by detecting and imaging the neuronal oscillations that are coherent across the brain [7-11].
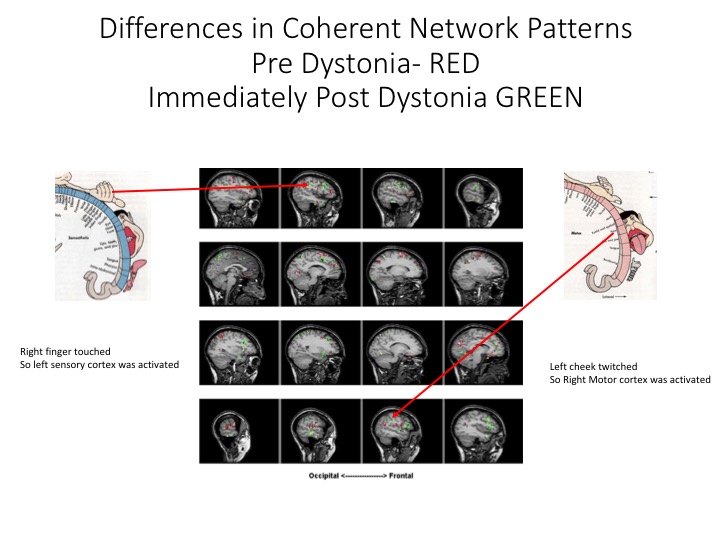
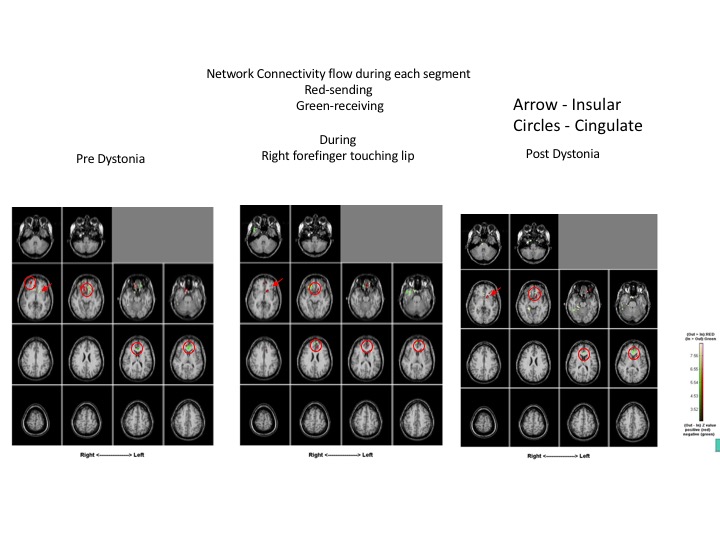
Results: Compared to normal control subjects, during rest, patient with ED displayed hyperexcitability and increased coherence diffusely in the bilateral parietal cortices as well as in the bilateral inferior frontal regions. Highly coherent networks became more focal within the bilateral sensory cortices [Figure 1] and motor cortex was activated when the patient touched her bottom lip, inducing the dystonic tremor [Figure 2]. Increased gamma frequencies were also found. After the finger was removed, hyperexcitability and increased coherence persisted in the bilateral parietal regions as well as the bilateral frontal regions. Pre-dystonia, the cingulate and insular gyri are receiving information while the right inferior frontal is transmitting information. During the dystonic activity, the right inferior frontal region is not significantly activated while the cingulate and insular gyri have both switched to transmitting information, something which persisted in the post-dystonic phase [Figure 3].
Conclusion: It may be postulated that increased coherence in the fronto-parietal regions at baseline as well as during dystonic activity reflect decreased intracortical inhibition and abnormal sensorimotor processing in ED. Increased alpha, beta and gamma-band frequencies during dystonic activity implicate abnormal pallido-cerebellar and cortico-pallidal pathways [12] while increased gamma following dystonia may be related to increased GABAergic activity in these regions [13].
References: 1. Frucht, S. Embouchure Dystonia-Portrait of a Task-Specific Cranial Dystonia. Movement Disorders 2009, 24, 1752-1762. 2. Sussman, J. Musician’s Dystonia. J Pract Neurol 2015, 15, 317-322. 3. Elbert, T.; Candia, E.; Altermuller, E. et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport 1998, 16, 3571-3575. 4. Mantel, T.; Dresel, C.; Altenmuller, E. et al. Activity and Topographic Changes in Somatosensory System in Embouchure Dystonia. Movement Disorders 2016, 31, 1640-1648. 5. Haslinger, B.; Altenmuller, E.; Castrop, F et al. Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology 2017, 74, 1790-1797. 6. Hirata, Y.; Schulz M.; Altenmuller, E. et al. Sensory mapping of the lip representation in brass musicians with embouchure dystonia. Neuroreport 2004, 15, 815-818. 7. Elisevich, K.; Shukla, N.; Moran, J. et al. An assessment of MEG coherence imaging in the study of temporal lobe epilepsy. Epilepsia 2011, 52, 1110-1119. 8. Nazem-Zadeh M.; Bowyer, S.; Zillgitt, A. et al. MEG coherence and DTI connectivity in mTLE. Brian Topography 2016, 29, 598-622. 9. Lajiness-O’Neill, R.; Richard, A.; Moran, J. et al. Neural synchrony examined with magnetoencephalography (MEG) during eye gaze processing in autism spectrum disorders: preliminary findings. Journal of Neurodevelopmental Disorders 2014, 6:15. 10. Mahajan, A.; Zillgitt, A.; Bowyer, S. et al. Sensory Trick in a Patient with Cervical Dystonia: Insights from Magnetoencephalography. Brain Sciences 2018, 8, 51. 11. Mahajan, A.; Alshammaa, A.; Zillgitt, A. et al. The effect of Botulinum Toxin on Network Connectivity in Cervical Dystonia: Lessons from Magnetoencephalography. Tremor and Other Hyperkinetic Movements 2017, 7, doi: 10/7916/D84M9H4W. 12. Neumann, W.; Ashwani, J.; Bock, A. et al. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 2015, 138, 1894-1906. 13. Muthukumaraswamy, S.; Edden, R.; Jones, D. et al. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. PNAS 2009, 106, 8356-8361.
To cite this abstract in AMA style:
B. Bulica, C. Sidiropoulos, A. Mahajan, A. Zillgitt, S. Bowyer. Embouchure dystonia evoked with tactile stimulation recorded by MEG [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/embouchure-dystonia-evoked-with-tactile-stimulation-recorded-by-meg/. Accessed December 16, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/embouchure-dystonia-evoked-with-tactile-stimulation-recorded-by-meg/