Session Information
Date: Tuesday, September 24, 2019
Session Title: Rating Scales
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To assess the feasibility of developing a patient-reported Botulinum Toxin Response Scale (BTRS) for different neurological conditions.
Background: Botulinum toxin (BoNT) is a highly effective symptomatic intramuscular therapy for treatment of a diverse group of disabling neurological disorders[1]. In the absence of any patient-reported scale for capturing improvement in symptoms and disability from BoNT treatment, current clinical practice of asking patients to recall this information clinic is limited and suffers from incomplete recall and recall bias. In addition, there is increasing evidence on the need for a more flexible patient-centered inter-injection interval of BoNT treatments[2], the development of which requires a patient-reported scale.
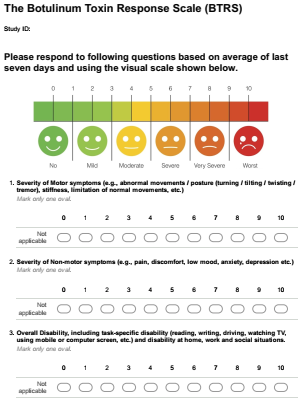
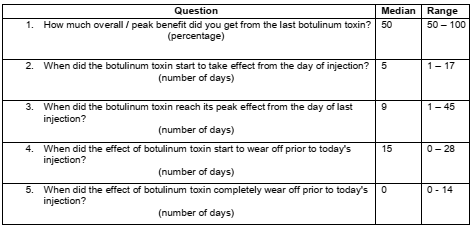
Method: Based on literature review of currently available clinical and patient-reported scales for application of botulinum toxin in dystonia, spasticity, blepharospasm and hemifacial spasm, as well as expert opinion (SP), a preliminary version of the BTRS was developed [figure1]. The BTRS was designed as a simple patient-reported scale for assessing severity of patients’ motor symptoms, non-motor symptoms and overall disability in preceding seven days. Subsequently, patients receiving BoNT treatment for neurological conditions were invited to participate in semi-structured interviews for capturing their responses on conventionally expected time course of benefit from BoNT treatment [figure2] and to fill BTRS on the day of injection visit in clinic.
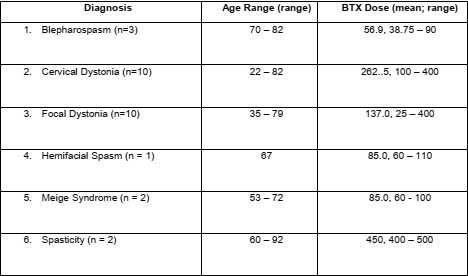
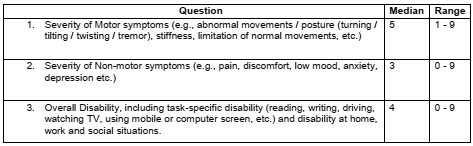
Results: A total of 28 patients (14 men and 14 women) with different diagnoses [table1] participated in the study. Patients’ responses varied widely on different questions pertaining to the expected time course of benefit from BoNT treatment [table2] and on the BTRS [table3]. The majority (26 out of 28, 92.9%) patient agreed that BTRS will potentially be a useful scale to them and / or their clinicians.
Conclusion: The results of this study support the need of developing the BTRS as a potentially useful patient-reported scale for improving the clinical practice of BoNT for neurological disorders.
References: 1. Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, Armstrong MJ, Gloss D, Potrebic S, Jankovic J. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818-26. 2. Wissel J. Towards flexible and tailored botulinum neurotoxin dosing regimens for focal dystonia and spasticity–Insights from recent studies. Toxicon. 2018;147:100-6.
To cite this abstract in AMA style:
D. Gupta, C. Gao, S. Pullman. Development of a patient-reported Botulinum Toxin Response Scale (BTRS) for neurological disorders: a feasibility study. [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/development-of-a-patient-reported-botulinum-toxin-response-scale-btrs-for-neurological-disorders-a-feasibility-study/. Accessed December 31, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/development-of-a-patient-reported-botulinum-toxin-response-scale-btrs-for-neurological-disorders-a-feasibility-study/