Session Information
Date: Tuesday, September 24, 2019
Session Title: Parkinsonisms and Parkinson-Plus
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To evaluate the safety, tolerability and pharmacokinetics of PBT434 in healthy volunteers.
Background: PBT434 is a novel, small molecule inhibitor of alpha-synuclein aggregation. In transgenic animal models of Parkinson disease (A53T) and Multiple System Atrophy (MSA) (PLP-alpha-Syn), PBT434 reduced alpha-synuclein aggregation, preserved neurons and improved motor function [1] [2]. Glial cell inclusions were also reduced in the MSA model. PBT434 is thought to act by redistributing reactive iron across membranes, thereby inhibiting intracellular protein aggregation and oxidative stress. The affinity of PBT434 for iron is greater than that of alpha-synuclein but lower than that of iron trafficking proteins such as ferritin.
Method: In this randomized, double-blind, placebo-controlled study, subjects received oral single ascending doses of 50 to 600 mg (SAD; n=8) or 8 days dosing with multiple ascending doses of 100 and 250 mg BID (MAD; n=10) of PBT434. Serial plasma samples were collected over 72 hours post-dose in the SAD phase and over 12- and 96-hours post-dose on Days 1 and 8, respectively, in the MAD phase. Drug levels were quantified from CSF sampled at 1.5 hours post-dose on Day 7 at the 250 mg BID dose. Safety was assessed with physical exam, vital signs, adverse events, laboratory tests and 12-lead ECGs.
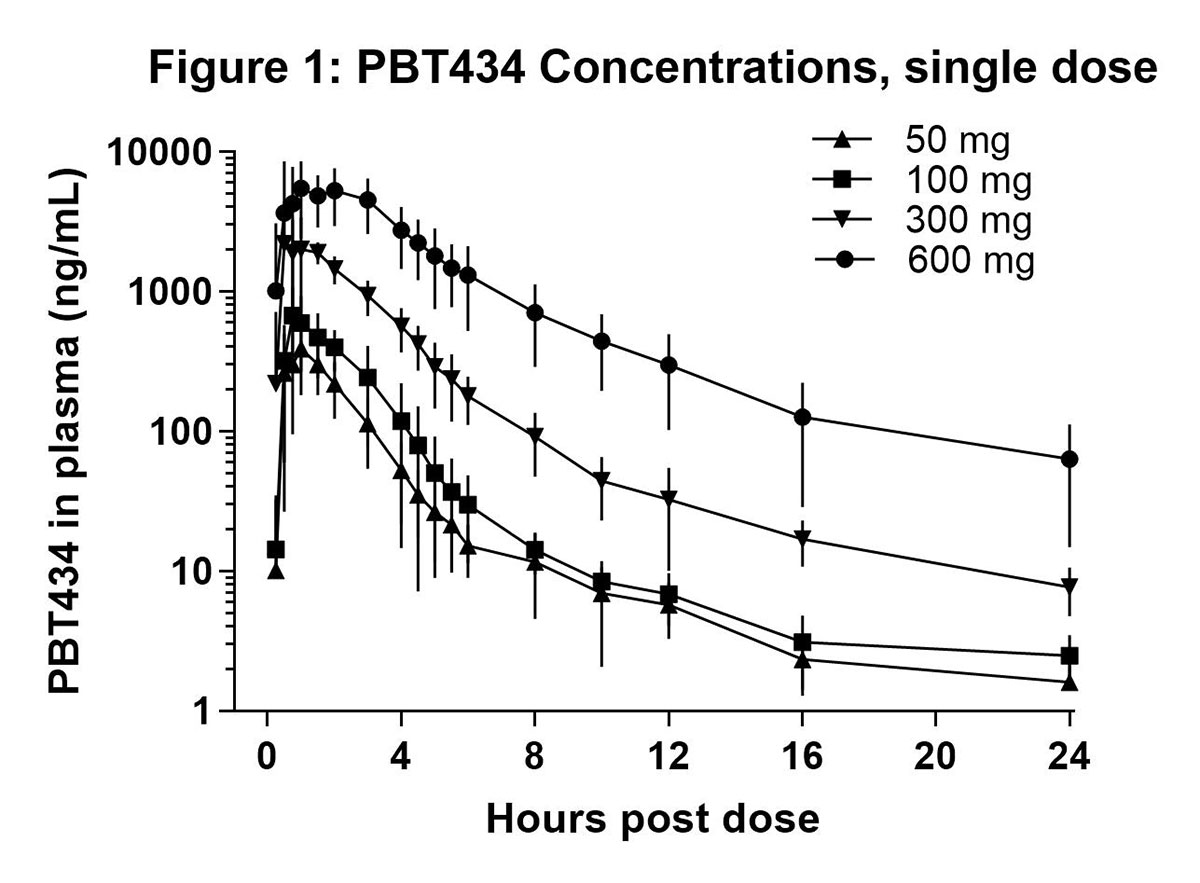
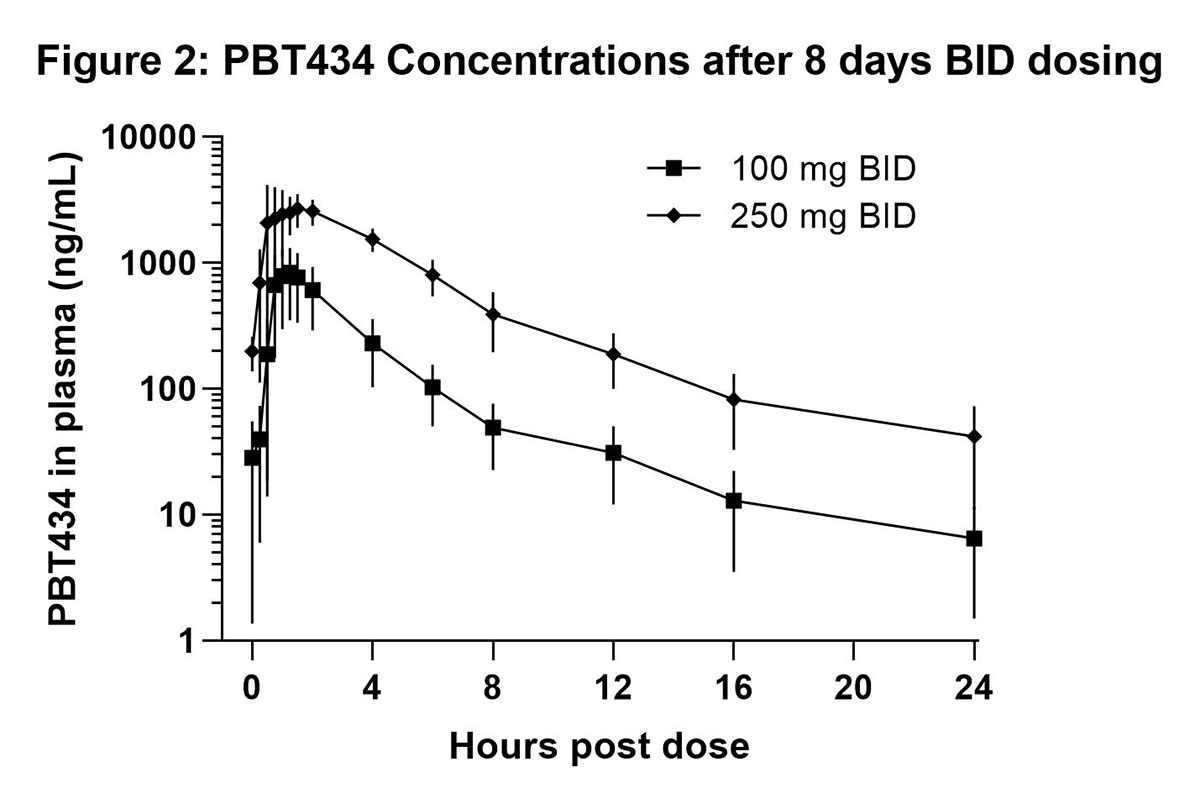
Results: PBT434 demonstrated dose dependent pharmacokinetics after single doses of 50 to 600 mg [figure 1], with a mean elimination half-life up to 9.3 hrs, and after 8 days dosing at 100 mg BID and 250 mg BID [figure 2]. PBT434 was rapidly and extensively absorbed after oral administration with a median Tmax of 1 to 1.25 hours and steady state was achieved by Day 7. At plasma Cmax, PBT434 exhibited good distribution into the CNS, achieving a mean (SD) CSF concentration of 79% (20) of the corresponding free plasma concentration. PBT434 was well tolerated with infrequent adverse events that were mild to moderate in severity. No serious adverse events were reported. No significant laboratory or ECG abnormalities were detected.
Conclusion: Single and repeated doses of PBT434 were well tolerated. PBT434 is a CNS-penetrant, orally bioavailable small molecule drug candidate with potential for treating synucleinopathies such as Parkinson disease and Multiple System Atrophy.
References: [1] Finkelstein DI, Billings JL, Adlard PA, et al. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol Commun. 2017;5(1):53. doi:10.1186/s40478-017-0456 2. [2] D. Finkelstein, P. Adlard, N. Stefanova, D. Stamler. PBT434 prevents the accumulation of glial cell inclusions and insoluble alpha-synuclein in a mouse model of Multiple System Atrophy. Mov Disord, Vol. 33, Suppl. 2, 2018. Abstract 990.
To cite this abstract in AMA style:
D. Stamler, M. Bradbury, C. Wong, E. Offman. A First in Human Study of PBT434, a Novel Small Molecule Inhibitor of Alpha-Synuclein Aggregation [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/a-first-in-human-study-of-pbt434-a-novel-small-molecule-inhibitor-of-alpha-synuclein-aggregation/. Accessed January 7, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-first-in-human-study-of-pbt434-a-novel-small-molecule-inhibitor-of-alpha-synuclein-aggregation/