Session Information
Date: Tuesday, September 24, 2019
Session Title: Parkinsonisms and Parkinson-Plus
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: The objective of this study to evaluate the efficacy and safety of subcutaneous apomorphine for patients with Parkinson’s disease (PD).
Background: Subcutaneous apomorphine has been widely used therapy for PD. However, there is limited evidence related to effectiveness in improving outcomes in patients with PD.
Method: A systematic search on MEDLINE and Cochrane Central Register of Controlled Trials was performed to identify English language articles published between January 2000 to January 2019. The eligible randomized controlled trials (RCTs) compared subcutaneous apomorphine with placebo as the control group for improving impairment, disability and health-related quality of in patients with PD. A random effects model was used to calculate the pooled mean difference (MD) and odds ratio (OR) with 95% confidence interval (CI). Meta-analysis was performed using RevMan 5.3 software. To test the robustness of the results, a sensitivity analysis was conducted by sequentially removing each study and reanalyzing the remaining studies.
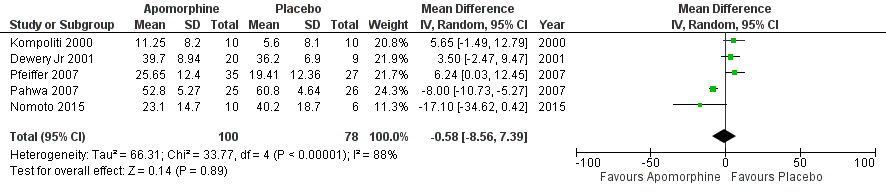
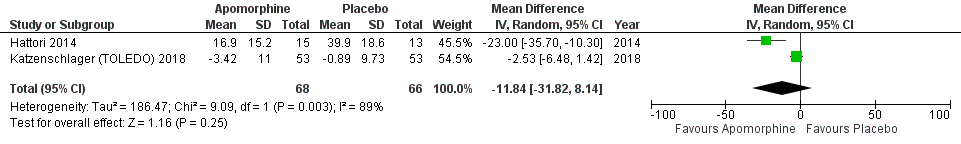
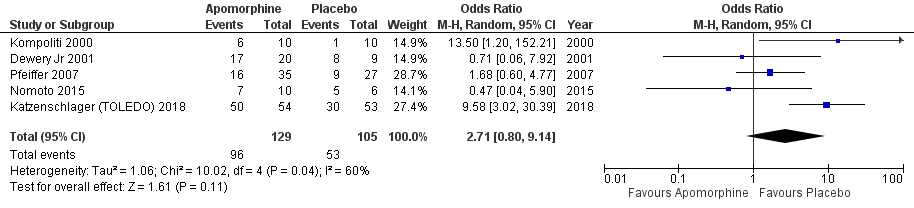
Results: A total of 7 RCTs included with 312 participants. The primary outcome measured by the Unified Parkinson’s Disease Rating Scale (UPDRS). Results from pooled meta-analysis showed evidence for no effect of subcutaneous apomorphine compared to control group on total UPDRS score (MD -0.58, 95% CI -8.56 to 7.39; P=0.89). A study observed a significant reduction in off time (hours) compared with the control group (1.89 hours per day, 95% CI -3.16 to -0.62 P< 0.002). However, there was no significant differences observed in UPDRS III (P=0.25) and the quality of life (QoL); PDQ 8 (P=0.34) scores. In addition, no significant difference was found in the adverse effects (OR 2.71, 95% CI 0.80 to 9.14; P=0.11). The sensitivity analysis revealed that two studies have substantial influence on the effect size for the significant changes in the score of UPDRS (MD -8.27, 95% CI -11.28 to -5.26; P=0.001).
Conclusion: The current meta-analysis found, a non-significant effect in improving QoL, disability, and impairment in patients with PD between subcutaneous apomorphine and control group. Although the sensitivity analysis found a significant effect of subcutaneous apomorphine on UPDRS when compared with the control group. Further long term follow-up RCTs and real-world studies required to make this finding more robust.
References: 1. Nomoto M, Kubo SI, Nagai M, Yamada T, Tamaoka A, Tsuboi Y, Hattori N, PD Study Group. A randomized controlled trial of subcutaneous apomorphine for Parkinson disease: a repeat dose and pharmacokinetic study. Clinical neuropharmacology. 2015 Nov 1;38(6):241-7. 2. Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet Neurology. 2018 Sep 1;17(9):749-59.
To cite this abstract in AMA style:
M. Azharuddin, M. Adil, P. Ghosh, M. Sharma. The Efficacy and Safety of Subcutaneous Apomorphine in Patients with Parkinson’s Disease: A Meta-Analysis Randomized Controlled Trials [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/the-efficacy-and-safety-of-subcutaneous-apomorphine-in-patients-with-parkinsons-disease-a-meta-analysis-randomized-controlled-trials/. Accessed April 2, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-efficacy-and-safety-of-subcutaneous-apomorphine-in-patients-with-parkinsons-disease-a-meta-analysis-randomized-controlled-trials/