Session Information
Date: Monday, September 23, 2019
Session Title: Rare Genetic and Metabolic Diseases
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To report the range of movement disorders associated with a NUS1 gene mutation.
Background: To date, pathogenic loss-of-function de novoNUS1mutations have been described in only 3 cases with developmental and epileptic encephalopathy (DEE). Movement disorders description in previous cases has been limited to either ataxia or tremors. We report a novel mutation of NUS1 gene presenting with movement disorders.
Method: Case report
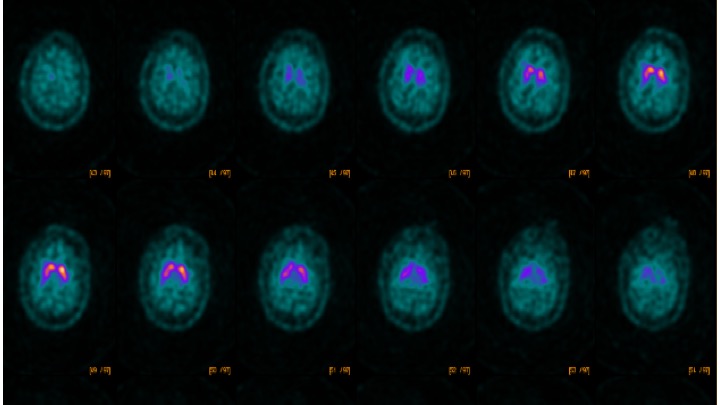
Results: A 53-year-old man presented with cognitive delay, childhood onset seizures, and abnormal body movements. Age of onset for seizures was 3-4 years and semiology consisted of absence, atonic and generalized tonic clonic seizures. Patient remains seizure free on combination of levetiracetam, clonazepam and phenobarbital. Pertinent neurological exam findings included cognitive impairment, dysarthria, impaired saccades, spasticity of legs with grade 4 hyperreflexia, action myoclonus of arms, facial myoclonus and mild appendicular ataxia with intentional tremors. MRI Brain showed mild global atrophy and DaTscan [figure1] was abnormal with reduced uptake in the right putamen. Whole exome sequencing revealed the previously unreported (absent in gnomAD) heterozygous truncating mutation c.99dupG; p.Asn34Glufs*100 in NUS1. This mutation was absent in his mother, while father’s DNA was unavailable for segregation studies.
Conclusion: Our case confirms NUS1 mutations as a cause of DEE and expands the spectrum of movement disorders associated with this condition. Our patient presents with ataxia, intentional tremors and prominent action myoclonus of arms and facial myoclonus, likely due to cortical and cerebellar involvement. We also demonstrate evidence of nigrostriatal degeneration, an interesting finding in light of a recent report of NUS1 as possible candidate gene for early onset Parkinson’s disease. NUS1 encodes for Nogo-B receptor (NgBR) which along with dehydrodolichyl diphosphate synthase (DHDDS) are critical for protein glycosylation. NgBR has also been shown to stabilize the nascent lysosomal NPC2 protein and regulate intracellular cholesterol trafficking. Functional studies in patient’s fibroblasts are ongoing to explore whether NUS1mutation lead to abnormal glycosylation and maturation of NPC2 and other lysosomal proteins with lysosomal storage abnormality as underlying pathology. The abstract will be presented at the AAN 71st annual meeting in May 2019.
References: [1] F.F. Hamdan, C.T. Myers, P. Cossette, P. Lemay, D. Spiegelman, A.D. Laporte, C. Nassif, O. Diallo, J. Monlong, M. Cadieux-Dion, S. Dobrzeniecka, C. Meloche, K. Retterer, M.T. Cho, J.A. Rosenfeld, W. Bi, C. Massicotte, M. Miguet, L. Brunga, B.M. Regan, K. Mo, C. Tam, A. Schneider, G. Hollingsworth, D.R. FitzPatrick, A. Donaldson, N. Canham, E. Blair, B. Kerr, A.E. Fry, R.H. Thomas, J. Shelagh, J.A. Hurst, H. Brittain, M. Blyth, R.R. Lebel, E.H. Gerkes, L. Davis-Keppen, Q. Stein, W.K. Chung, S.J. Dorison, P.J. Benke, E. Fassi, N. Corsten-Janssen, E.J. Kamsteeg, F.T. Mau-Them, A.L. Bruel, A. Verloes, K. Õunap, M.H. Wojcik, D.V.F. Albert, S. Venkateswaran, T. Ware, D. Jones, Y.C. Liu, S.S. Mohammad, P. Bizargity, C.A. Bacino, V. Leuzzi, S. Martinelli, B. Dallapiccola, M. Tartaglia, L. Blumkin, K.J. Wierenga, G. Purcarin, J.J. O’Byrne, S. Stockler, A. Lehman, B. Keren, M.C. Nougues, C. Mignot, S. Auvin, C. Nava, S.M. Hiatt, M. Bebin, Y. Shao, F. Scaglia, S.R. Lalani, R.E. Frye, I.T. Jarjour, S. Jacques, R.M. Boucher, E. Riou, M. Srour, L. Carmant, A. Lortie, P. Major, P. Diadori, F. Dubeau, G. D’Anjou, G. Bourque, S.F. Berkovic, L.G. Sadleir, P.M. Campeau, Z. Kibar, R.G. Lafrenière, S.L. Girard, S. Mercimek-Mahmutoglu, C. Boelman, G.A. Rouleau, I.E. Scheffer, H.C. Mefford, D.M. Andrade, E. Rossignol, B.A. Minassian, J.L. Michaud, High Rate of Recurrent De Novo Mutations in Developmental and Epileptic Encephalopathies, Am. J. Hum. Genet. 101 (2017) 664–685. doi:10.1016/j.ajhg.2017.09.008. [2] F. Zhang, X. Yan, J. Xue, Q. Sun, J. Chen, B. Tang, J. Mei, Y. Zhao, L. Zhang, Z. Hu, X. Zhu, X. Tang, G. Wang, H. Guo, H. Cai, Z. Zhu, Y. Jiang, S.C. Li, J. Liu, G. Zhao, K. Xia, H. Shang, P. Chan, Z. Yue, C. Liu, X. Wu, X. Xie, R. Duan, Z. Liu, S. Sun, J. Guo, K. Li, Z. Zhang, H. Jiang, J. Li, Q. Xu, X. Fang, C. Chen, T. Wang, L. Shen, Coding mutations in NUS1 contribute to Parkinson’s disease , Proc. Natl. Acad. Sci. 115 (2018) 11567–11572. doi:10.1073/pnas.1809969115. [3] K.D. Harrison, R.Q. Miao, C. Fernandez-Hernándo, Y. Suárez, A. Dávalos, W.C. Sessa, Nogo-B Receptor Stabilizes Niemann-Pick Type C2 Protein and Regulates Intracellular Cholesterol Trafficking, Cell Metab. 10 (2009) 208–218. doi:10.1016/j.cmet.2009.07.003. [4] E.J. Park, K.A. Grabińska, Z. Guan, V. Stránecký, H. Hartmannová, K. Hodaňová, V. Barešová, J. Sovová, L. Jozsef, N. Ondrušková, H. Hansíková, T. Honzík, J. Zeman, H. Hůlková, R. Wen, S. Kmoch, W.C. Sessa, Mutation of Nogo-B receptor, a subunit of cis-prenyltransferase, causes a congenital disorder of glycosylation, Cell Metab. 20 (2014) 448–457. doi:10.1016/j.cmet.2014.06.016.
To cite this abstract in AMA style:
N. Prakash, N. Mencacci, C. Zadikoff, L. Kinsley, T. Simuni, S. Lubbe, D. Krainc. Novel Mutation of NUS1 Gene Presenting With Developmental And Epileptic Encephalopathy and Movement Disorders [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/novel-mutation-of-nus1-gene-presenting-with-developmental-and-epileptic-encephalopathy-and-movement-disorders/. Accessed December 23, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/novel-mutation-of-nus1-gene-presenting-with-developmental-and-epileptic-encephalopathy-and-movement-disorders/