Session Information
Date: Monday, September 23, 2019
Session Title: Genetics
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To describe the clinical picture of a patient with a large deletion in band 8p11.21p11.1 including SLC20A2 and present a review of patients with a SLC20A2 copy number variation (CNV), who show primary familial brain calcification.
Background: Primary familial brain calcification (PFBC; Fahr disease) is a rare progressive cerebral disorder with both movement disorders (parkinsonism, dystonia, ataxia, chorea) and neuropsychiatric disturbances (psychosis, dementia and frontal or subcortical cognitive dysfunction), often inherited as a dominant trait. Mutations in SLC20A2, PDGFB, PDGFRB, and XPR1 have been reported. Loss of function of either SLC20A2 or the PDGFB/PDGFRB pathway is the mechanism underlying calcification in patients with a mutation.
Method: A 28 year old male patient was referred for genetic counseling because of neurodegeneration with brain iron accumulation. He was known with a mild motor and intellectual disability and showed progressive deterioration of his cognitive function, a movement disorder with dystonia, tremor and sensory ataxia.
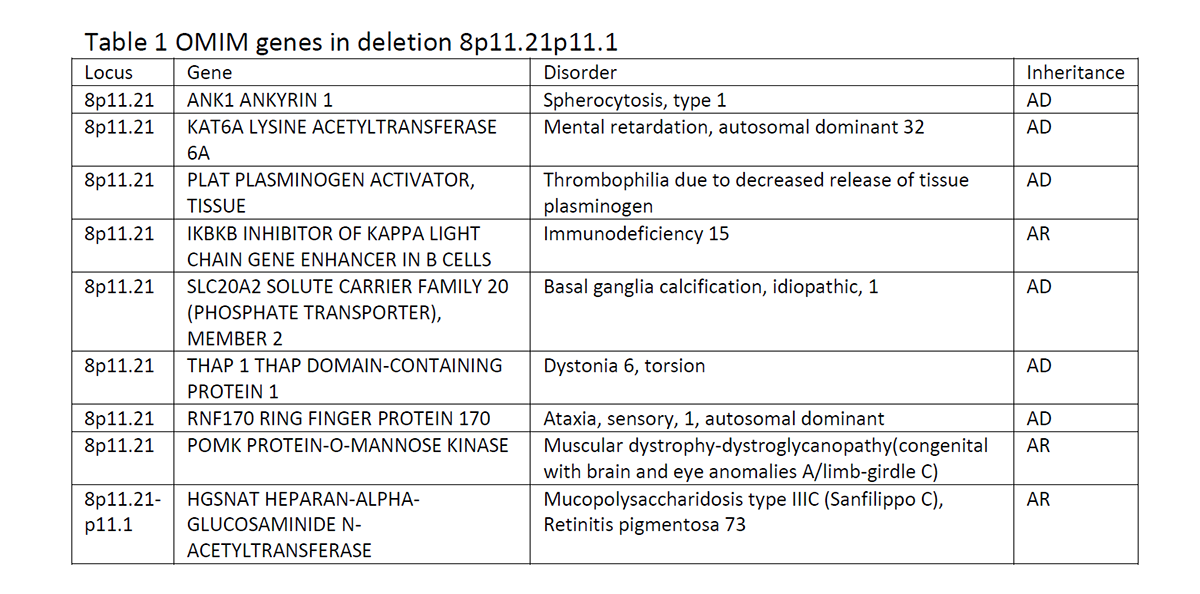
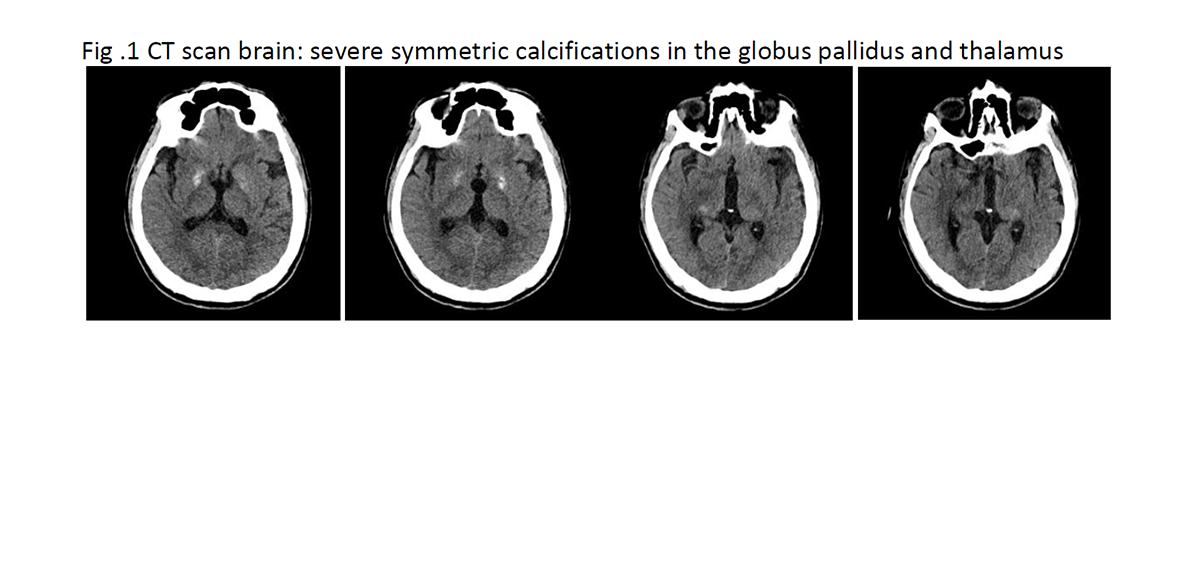
Results: A mutation in the NBIA genes was not detected by next generation sequencing. A SNP array showed a 3.3Mb loss of 8p11.21p11.1, including SLC20A2 (among others: Table 1) and this loss showed to be de novo. A CT-scan of the brain showed severe symmetric calcifications in the globus pallidus and thalamus, compatible with Fahr disease (Fig. 1). As loss of function of either SLC20A2 or the PDGFB/PDGFRB pathway is the mechanism underlying calcification in patients with a mutation, a deletion involving SLC20A2 can explain his clinical picture. The effect of the other genes in the deletion like THAP1, KAT6A and RNG170 could also contribute to the clinical picture, like dystonia, intellectual disability and sensory ataxia.
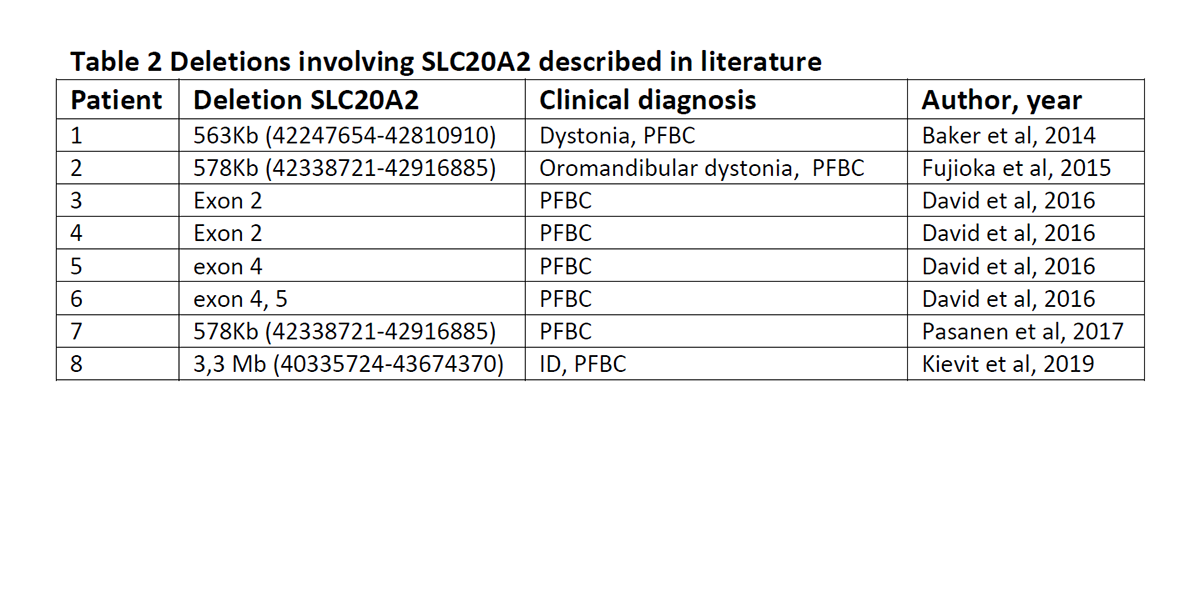
Conclusion: Reverse phenotyping by CNV analysis followed by further clinical workup lead to the diagnosis PFBC in this patient. CNVs should be investigated in PFBC patients with no point mutation in associated genes, because a rather high frequency (16.7%) of SLC20A2 deletions has been described (David et al, 2014). The 7 index patients with a CNV in SLC20A2 described before show PFBC, but other genes of the CNV can modify the clinical picture (Table 2).
References: Matt Baker & Audrey J. Strongosky & Monica Y. Sanchez-Contreras & Shan Yang &Will Ferguson & Donald B. Calne & Susan Calne & A. Jon Stoessl & Judith E. Allanson & Daniel F. Broderick & Michael L. Hutton & Dennis W. Dickson & Owen A. Ross &Zbigniew K. Wszolek & Rosa Rademakers. SLC20A2 and THAP1 deletion in familial basal ganglia calcification with dystonia. Neurogenetics (2014) 15:23–30 Stéphanie David, Joana Ferreira, Olivier Quenez, Anne Rovelet-Lecrux, Anne-Claire Richard,Marc Vérin, Snejana Jurici, Isabelle Le Ber, Anne Boland, Jean- François Deleuze, Thierry Frebourg,João Ricardo Mendes de Oliveira, Didier Hannequin, Dominique Campion and Gaël Nicolas. Identification of partial SLC20A2 deletions in primary brain calcification using whole-exome sequencing.European Journal of Human Genetics (2016) 24, 1630–1634 Shinsuke Fujioka,Audrey J. Strongosky,Anhar Hassan, Rosa Rademakers,Dennis W. Dickson, Zbigniew K. Wszolek. Clinical presentation of a patient with SLC20A2 and THAP1 deletions:Differential diagnosis of oromandibular dystonia.Parkinsonism and Related Disorders 21 (2015) 329-331 P. Pasanen, J. Mäkinen, L. Myllykangas, R. Guerreiro, J. Bras, M. Valori, M. Viitanen, M. Baumann, P. J. Tienari, M. Pöyhönen,P. Baumann. Primary familial brain calcification linked to deletion of 5’noncoding region of SLC20A2.Acta Neurol Scand. 2017;136:59–63.
To cite this abstract in AMA style:
A. Kievit, L. Zutven, A. Boon. Reverse phenotyping of a 3.3Mb loss of 8p11.21p11.1, including SLC20A2, lead to a diagnosis of primary familial brain calcification [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/reverse-phenotyping-of-a-3-3mb-loss-of-8p11-21p11-1-including-slc20a2-lead-to-a-diagnosis-of-primary-familial-brain-calcification/. Accessed October 21, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/reverse-phenotyping-of-a-3-3mb-loss-of-8p11-21p11-1-including-slc20a2-lead-to-a-diagnosis-of-primary-familial-brain-calcification/