Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Summarize improvement in quality of life (QoL) by comparing scores from the EQ-5D questionnaire, five level response version (EQ-5D-5L) summary index and visual analog scale (EQ-VAS) obtained for deep brain stimulation (DBS) therapy-naive PD patients from baseline through 3 years post-implant.
Background: EQ-5D index is a standardized, generic (disease non-specific) self-reporting instrument to evaluate the impact of treatment on health related QoL. When compared to disease specific instruments (e.g. PDQ-39 and SF-36), the EQ-5D-3L was a valid instrument to measure QoL in PD[1]. Our study continues to evaluate the effectiveness of DBS therapy for PD as measured by the EQ-5D-5L summary index and EQ-VAS in a large, global real-world population.
Method: The Product Surveillance Registry (PSR) is a prospective, long-term, multi-center registry originated for DBS in 2009 and represents data collected on over 2500 patients in the USA, Europe and Latin America. The EQ-5D-5L questionnaire was added to the PSR in 2013 and includes domains of mobility, self-care, usual activities, pain/discomfort, anxiety/depression. Analyses of the EQ-5D index value used the England value set, which ranges from 1 (best health state) to -0.285 (worst health state)[2]. The EQ-VAS assesses health state from 0 (worst health) to 100 (best health you can image) on the day of the visit. Within patient changes from baseline through 3 years were tested using the Wilcoxon signed rank test.
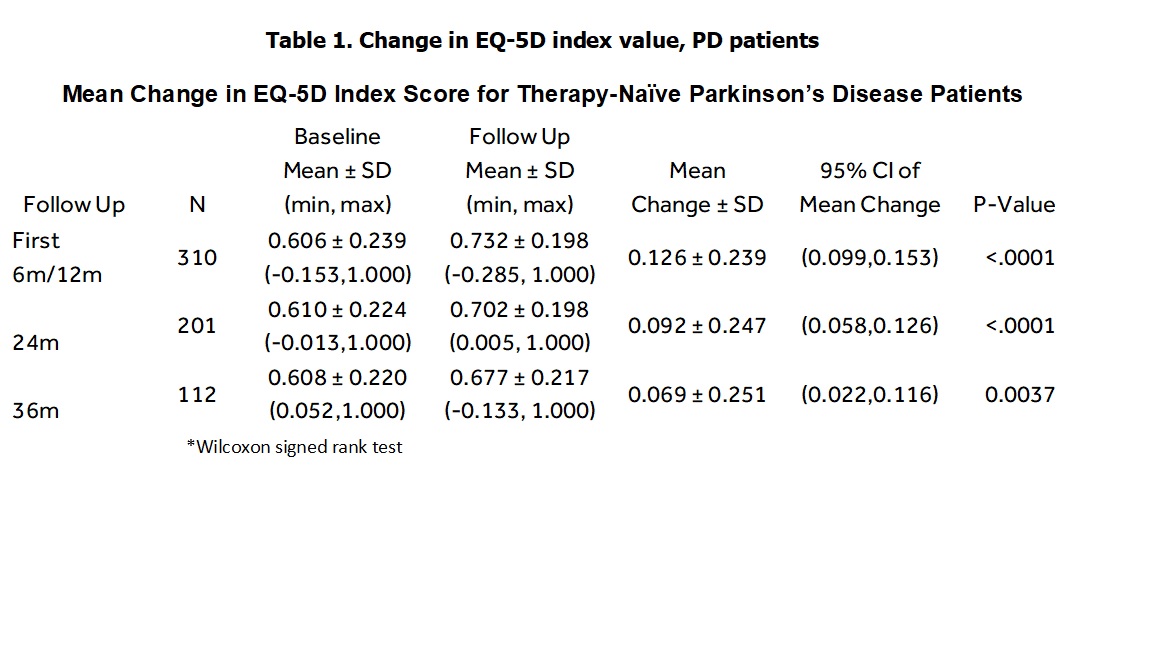
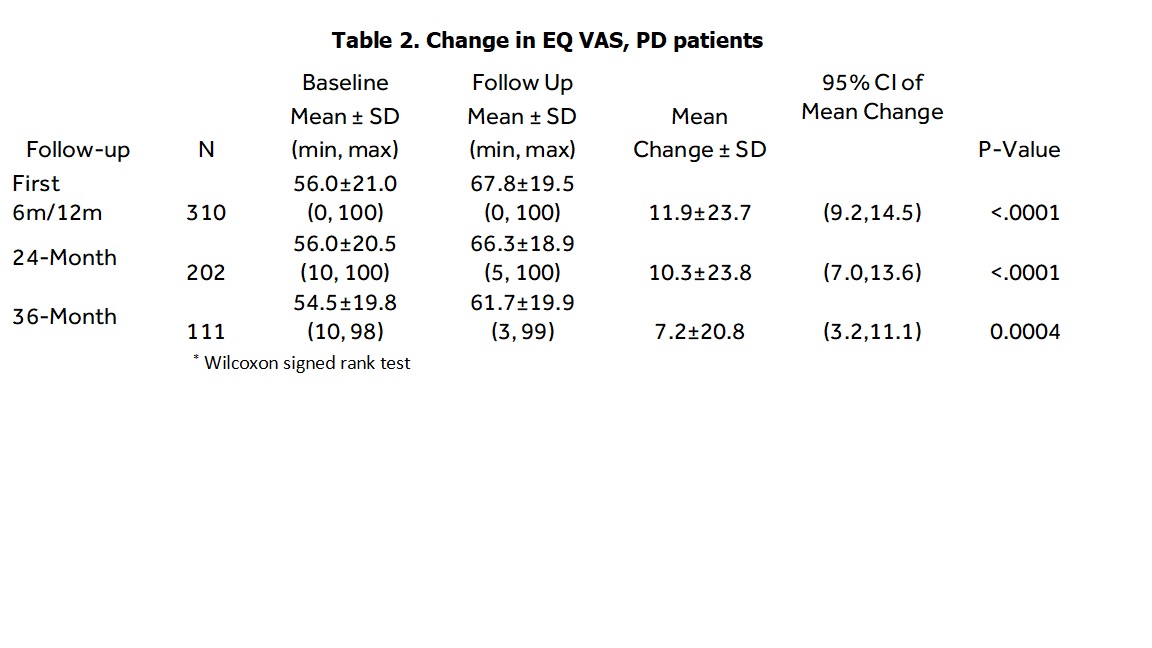
Results: A total of 332 therapy-naïve PD patients with at least one paired EQ-5D score available sometime between baseline and 3 years post-implant were included. Mean age at enrollment was 62.8 ± 8.9 years and 69.6% were male. Mean years from disease onset to DBS implant was 10.2 years ± 4.7 years. As shown in Tables 1-2, EQ-5D-5L summary index scores improved 0.126 (p<. 0001) at first follow-up (either 6 or 12-month), 0.092 at 2-years (p<. 0001), and 0.069 at 3-years (p=0.004). The EQ-VAS improved 11.9 units during the first follow-up, 10.3 at 2 years, and 7.2 at 3 years. The minimally important difference (MID) in the EQ-5D index score using the scoring algorithm has been estimated at 0.037[3]. [table 1] [table 2]
Conclusion: Results from the PSR demonstrated significant improvement in EQ-5D index score and VAS at 1, 2, and 3 years post-implant. Future analysis may evaluate the impact of DBS earlier or later in the treatment course on quality of life.
References: 1. Schrag A,Selai C, Jahanshahi M, Quinn NP. The EQ-5D – a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson’s disease. JNNP 2000; 69:67-73. 2. Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;Jan27(1):7-22. 3. McClure NS, Al Sayah F, Xie F, et al. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value in Health 2017;20:644-50
To cite this abstract in AMA style:
M. Schiess, S. Palfi, J. Azulay, A. Lopez Rios, H. Xiong, K. Sandberg, JK. Krauss. 3 Year QoL Results for PD Patients Receiving DBS: Product Surveillance Registry [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/3-year-qol-results-for-pd-patients-receiving-dbs-product-surveillance-registry/. Accessed December 28, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/3-year-qol-results-for-pd-patients-receiving-dbs-product-surveillance-registry/