Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To review the performance of UHDRS-TMS and Q-Motor quantitative motor measures in recent placebo-controlled clinical trials.
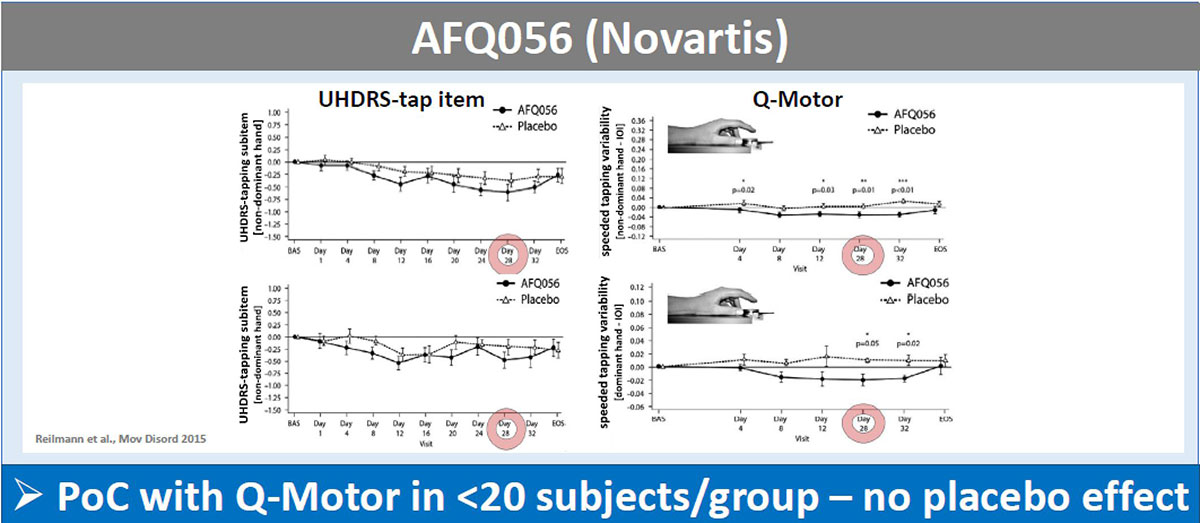
Background: The UHDRS-TMS has widely been used to assess motor symptoms in HD clinical trials – often serving as primary endpoint. Sizeable placebo effects in the UHDRS-TMS were observed. Recently objective assessment of motor function using the Q-Motor quantitative motor battery has been introduced in multiple HD clinical trials [1].
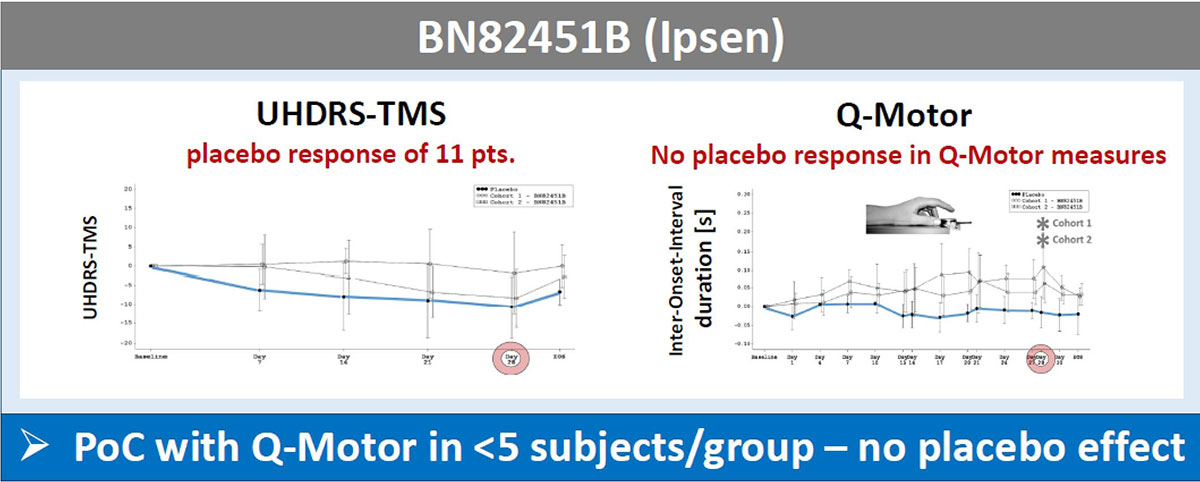
Method: Results of motor assessments of the studies AFQ056 (Novartis) [2], PRIDE-HD (Teva) [3], AMARYLLIS (Pfizer), LEGATO-HD (Teva), BN82451B (Ipsen) are compared and characteristic behavior of these measures are identified.
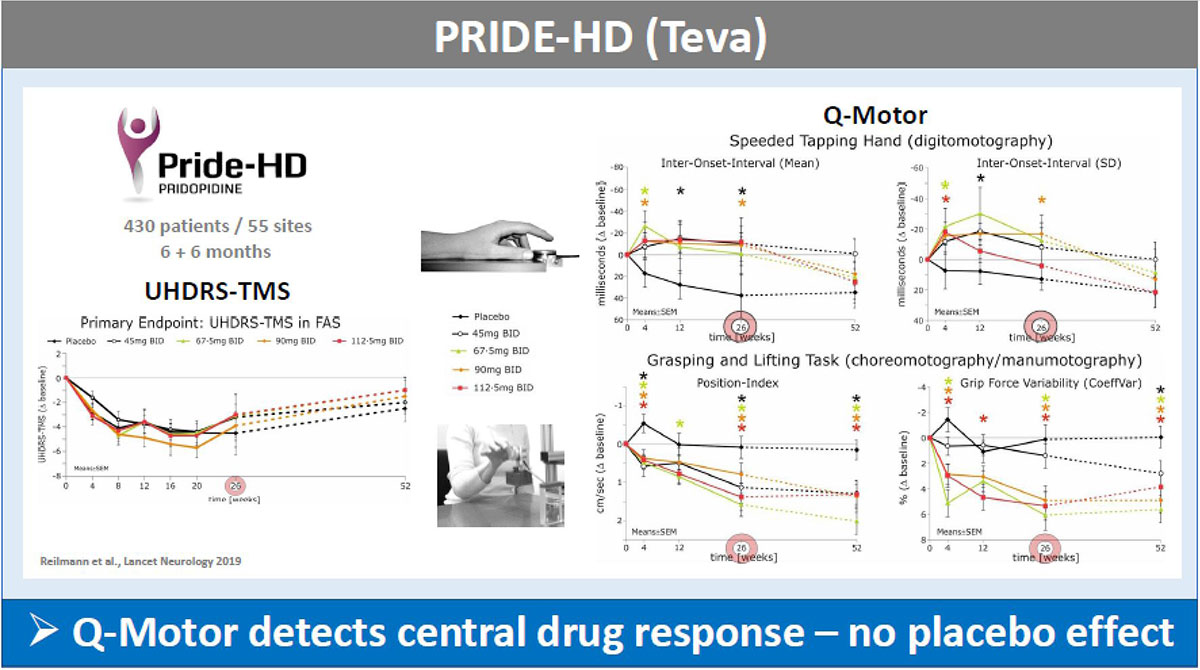
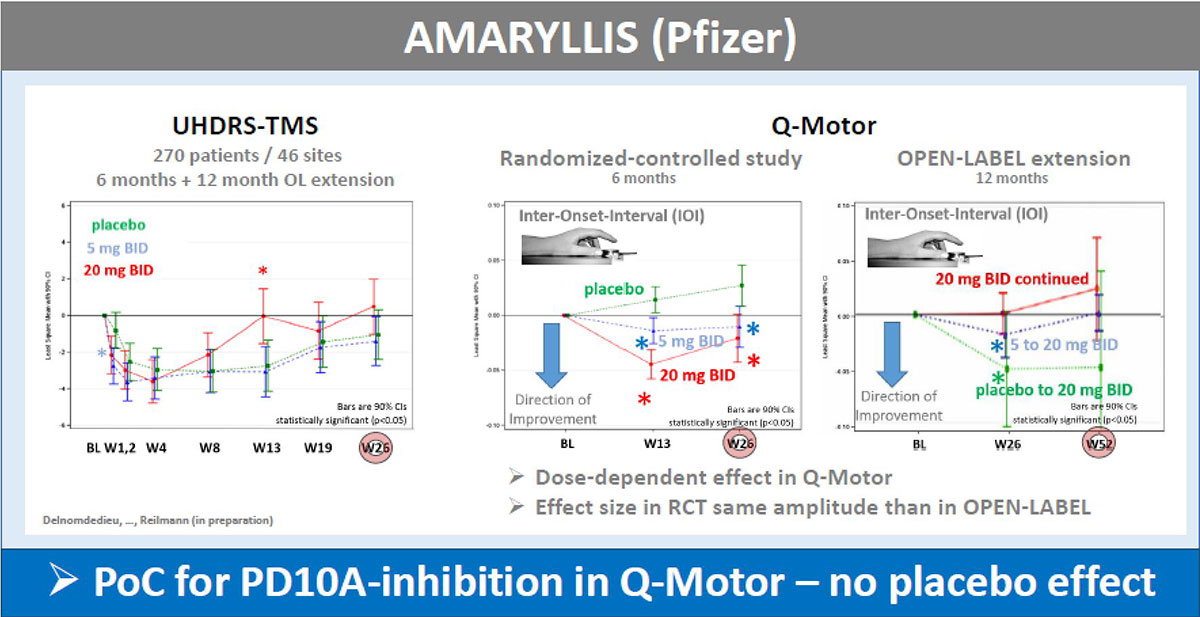
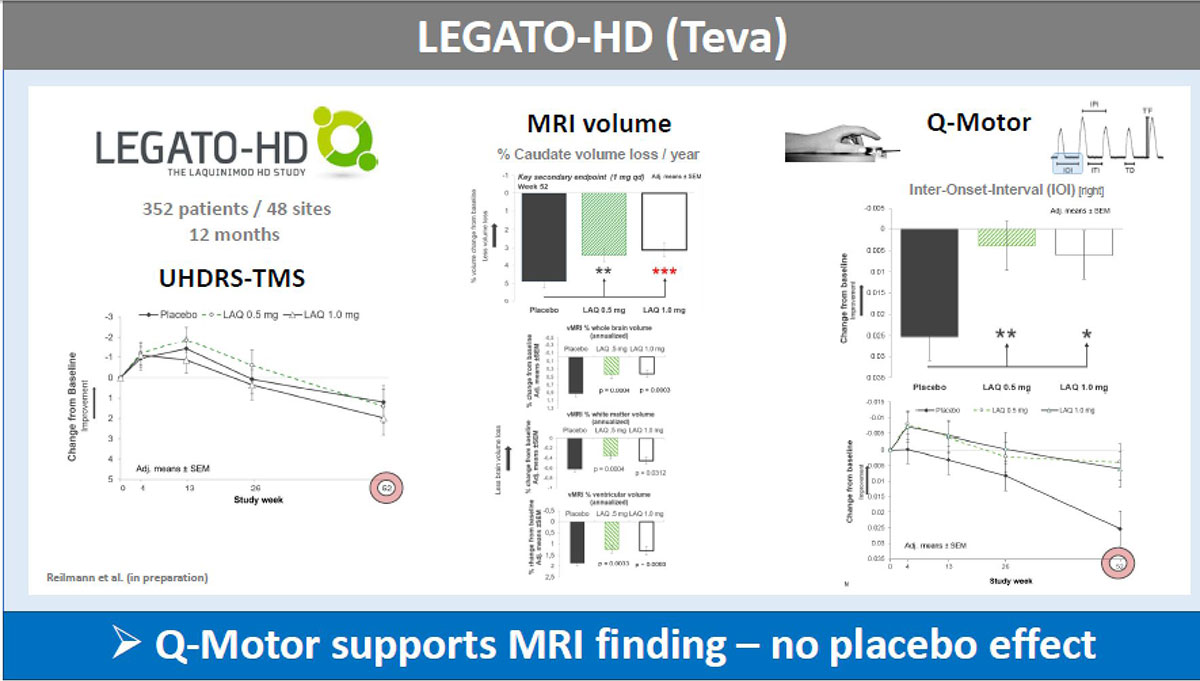
Results: The UHDRS-TMS (or sub-items) served as primary endpoint in all these trials; mean UDHRS-TMS scores of the placebo arm improved during all studies, while no treatment effects were seen. In contrast, Q-Motor measures remained stable in the placebo arm with low variability during the two shorter trials of four weeks duration and declined in longer trials over 6-12 months durations, as expected in HD patients. Q-Motor measures detected normally significant improvements in AFQ056, PRIDE-HD, AMARYLLIS (dose-dependent) and LEGATO-HD (with supporting MRI findings) [figure1][figure2][figure3][figure4]. In BN82451B and PRIDE-HD Q-Motor measures also detected deterioration of motor performance [figure5]. Of note, the dose-dependent mean effect sizes of Q-Motor benefits observed in AMARYLLIS were reproduced in the consecutive open label study. Q-Motor has been installed in > 140 sites globally and data was collected and analyzed reliably.
Conclusion: Q-Motor assessments provided a higher sensitivity than the UHDRS-TMS across multiple clinical trials. The lack of placebo responses in Q-Motor measures was observed consistently. This may largely be driven by the fact that Q-Motor measures are rater-independent and thus standardized. Proof-of-concept of drug effects in small patient cohorts and the potential to see similar effects in placebo controlled and open label settings merits further investigations. Innovative strategies to perform economic and de-risked clinical trials based on these findings will be discussed.
References: [1] Reilmann R, Schubert R (2017) Motor outcome measures in Huntington disease clinical trials. Handb Clin Neurol 144:209-225. doi: 10.1016/B978-0-12-801893-4.00018-3.:209-225. [2] Reilmann R, Rouzade-Dominguez ML, Saft C, Sussmuth SD, Priller J, Rosser A, Rickards H, Schols L, Pezous N, Gasparini F, Johns D, Landwehrmeyer GB, Gomez-Mancilla B (2015) A randomized, placebo-controlled trial of AFQ056 for the treatment of chorea in Huntington’s disease. Mov Disord 30:427-431. [3] Reilmann R, McGarry A, Grachev ID, Savola JM, Borowsky B, Eyal E, Gross N, Langbehn D, Schubert R, Wickenberg AT, Papapetropoulos S, Hayden M, Squitieri F, Kieburtz K, Landwehrmeyer GB (2019) Safety and efficacy of pridopidine in patients with Huntington’s disease (PRIDE-HD): a phase 2, randomised, placebo-controlled, multicentre, dose-ranging study. Lancet Neurol 18:165-176.
To cite this abstract in AMA style:
R. Reilmann, R. Schubert, P. Barallon, P. Pracht, B. Habbel. Motor assessments in HD clinical trials: Q-Motor versus the UHDRS-TMS – what did we learn from recent studies? [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/motor-assessments-in-hd-clinical-trials-q-motor-versus-the-uhdrs-tms-what-did-we-learn-from-recent-studies/. Accessed December 23, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/motor-assessments-in-hd-clinical-trials-q-motor-versus-the-uhdrs-tms-what-did-we-learn-from-recent-studies/