Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To evaluate safety and pharmacodynamic (PD) profiles of the first recombinant botulinum toxins (BoNT) serotype E (rBoNT-E) versus abobotulinumtoxinA (aboBoNT-A) in healthy male volunteers.
Background: Naturally occurring BoNT serotypes have different pharmacological features, which are of therapeutic and aesthetic interest. AboBoNT-A is an effective treatment for dystonias and limb spasticity, with a relatively fast onset and a long duration of action. A BoNT serotype with a faster onset and limited duration may be beneficial in certain circumstances.
Method: Double-blind, placebo-controlled, ascending-dose study (EudraCT: 2016-002609-20) in 28 healthy human males randomised (3:1) to receive rBoNT-E (sequential cohorts, up to 3.6ng rBoNT-E) or placebo. A further 24 subjects were randomised (6 per treatment arm) to receive a double-blind injection of aboBoNT-A 20, 40 or 70U, or placebo. All injections were into the right extensor digitorum brevis (EDB) muscle. PD profile was assessed by recording compound muscle action potential (CMAP) in injected EDB following supramaximal stimulation. Data from active treatment groups only (rBoNT-E and aboBoNT-A) are described.
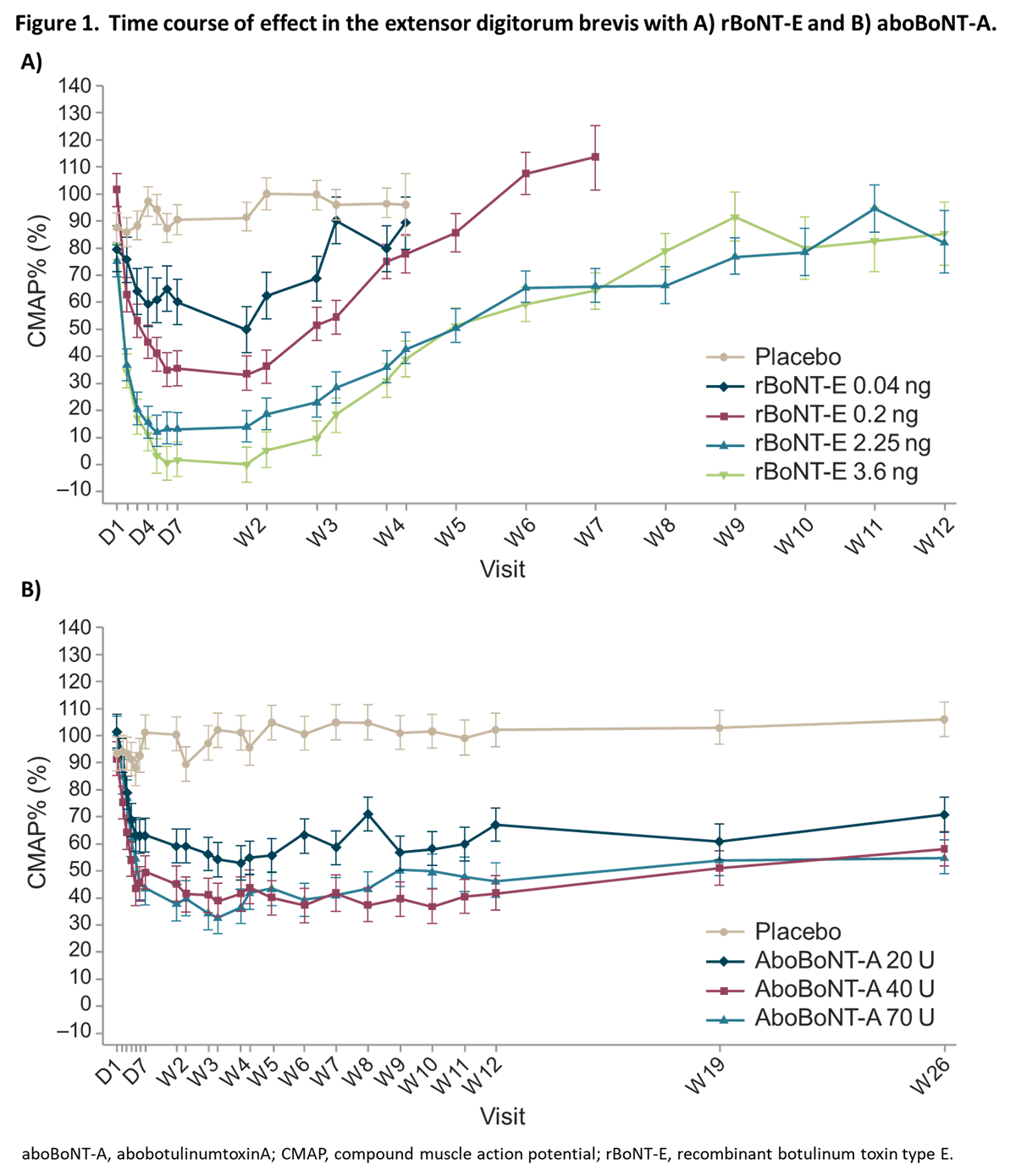
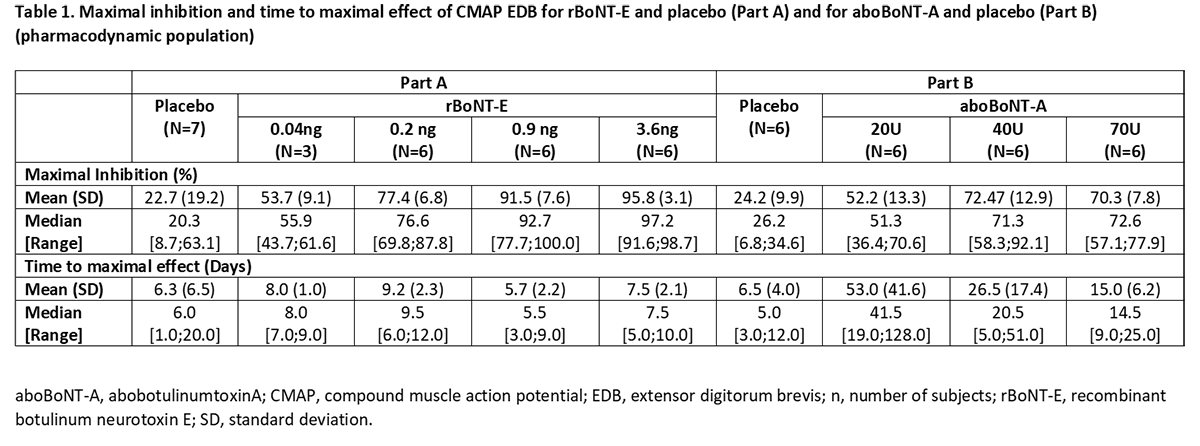
Results: All rBoNT-E doses were well tolerated. Most treatment-emergent adverse events were considered unrelated to treatment, and no unexpected treatment-related toxicities were identified with rBoNT-E or aboBoNT-A. Onset of action (15% inhibition of CMAP) occurred on Day 1 or 2 with rBoNT-E, and Day 1 to 7 with aboBoNT-A. Maximal CMAP inhibition was: ~50% for 0.04ng rBoNT-E and 20U aboBoNT-A; ~70% for 0.2ng rBoNT-E, 40 and 70U aboBoNT-A; and ~90% for 0.9 and 3.6ng rBoNT-E. Maximal effect was reached ~1 week post-injection for rBoNT-E and 2─6 weeks for aboBoNT-A. Inhibition lasted ~7 weeks for 0.9 and 3.6ng rBoNT-E, persisting until 26 weeks post-injection for aboBoNT-A subjects.
Conclusion: An overall good safety profile of single intramuscular doses of rBoNT-E was demonstrated up to 3.6ng. rBoNT-E had a faster onset of effect, greater and quicker peak effect and shorter duration of action versus aboBoNT-A, when injected in EDB muscles of healthy males. The PD profile of rBoNT-E addresses new and different patient needs in those with movement disorders.Data previously presented at TOXINS 2019 and IMCAS 2019. [Figure 1] [Table 1]
To cite this abstract in AMA style:
L. Pons, C. Vilain, P. Picaut. Outcomes of the first-in-human study with a recombinant botulinum toxin E (rBoNT-E): safety and pharmacodynamic profile of rBoNT-E compared with abobotulinumtoxinA [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/outcomes-of-the-first-in-human-study-with-a-recombinant-botulinum-toxin-e-rbont-e-safety-and-pharmacodynamic-profile-of-rbont-e-compared-with-abobotulinumtoxina/. Accessed April 1, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/outcomes-of-the-first-in-human-study-with-a-recombinant-botulinum-toxin-e-rbont-e-safety-and-pharmacodynamic-profile-of-rbont-e-compared-with-abobotulinumtoxina/