Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To evaluate the long-term safety and efficacy of apomorphine infusion (APO) in PD patients with motor fluctuations despite optimised oral therapy.
Background: The randomised, double-blind phase (DBP) of the TOLEDO study (NCT02006121) confirmed the efficacy of apomorphine infusion (APO) in significantly reducing OFF time versus placebo in PD patients with motor fluctuations despite optimised oral therapy.1 Here we report results including the 52-week open-label phase (OLP).
Method: All patients completing the DBP (including early switching) were offered OLP entry. The primary objective was evaluation of long-term safety of APO. Efficacy parameters were also collected.
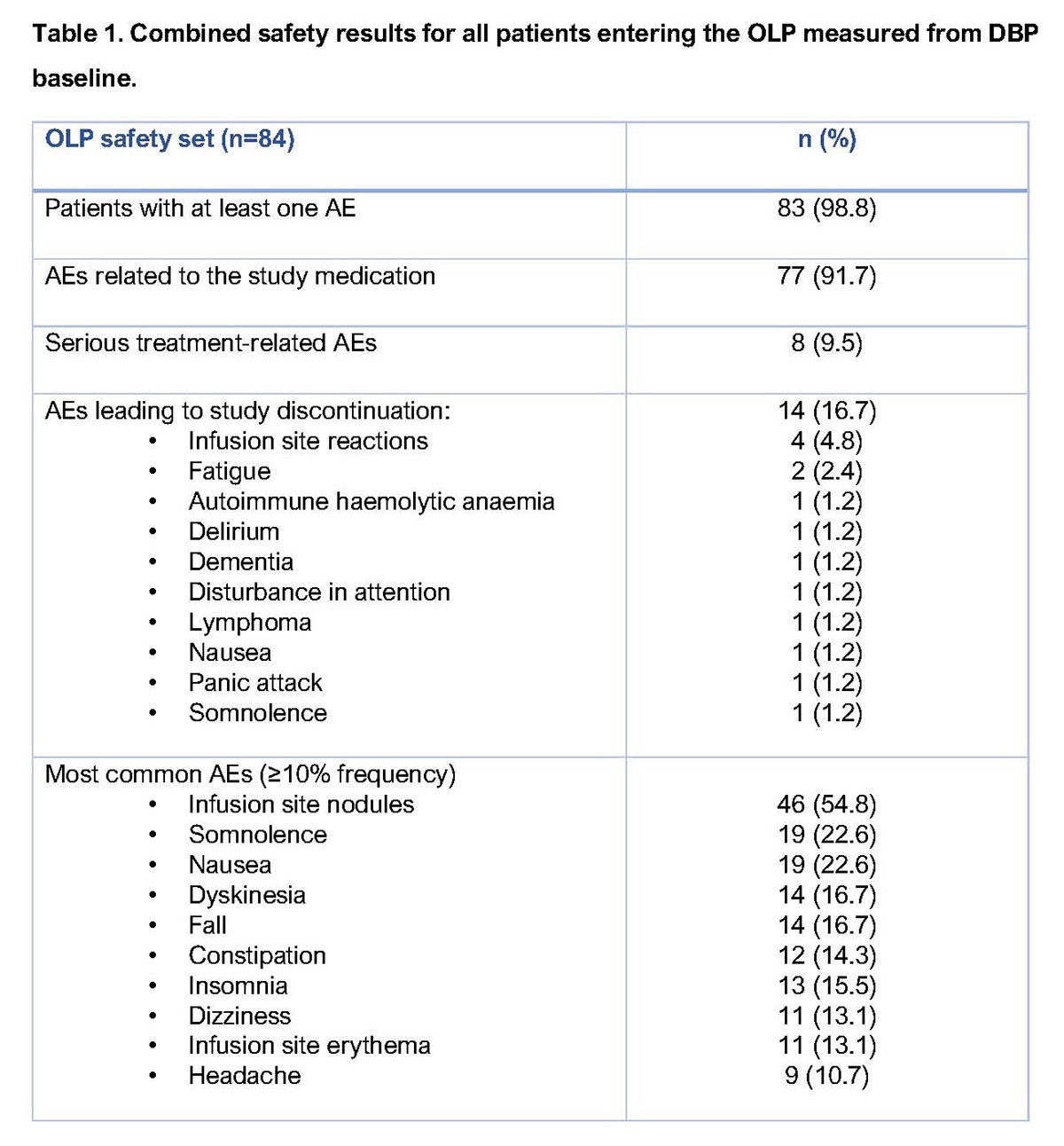
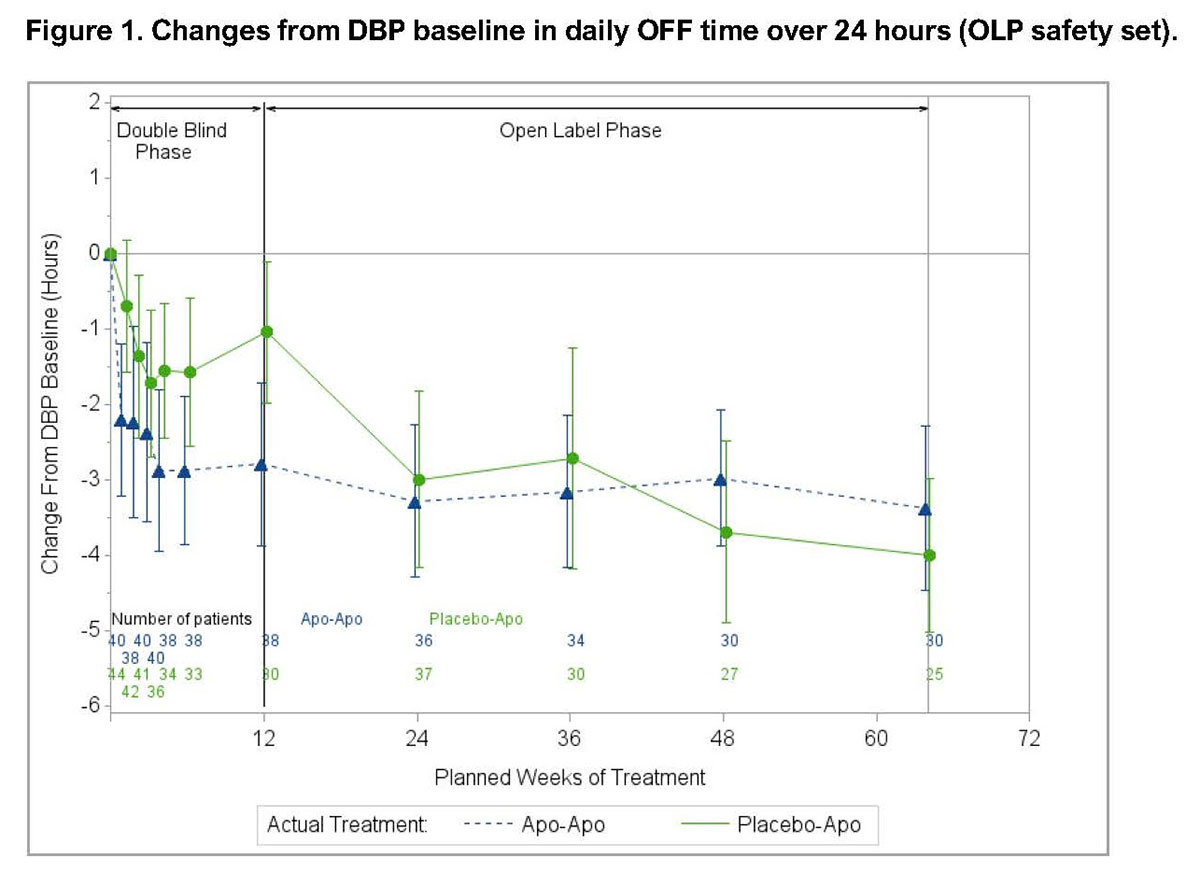
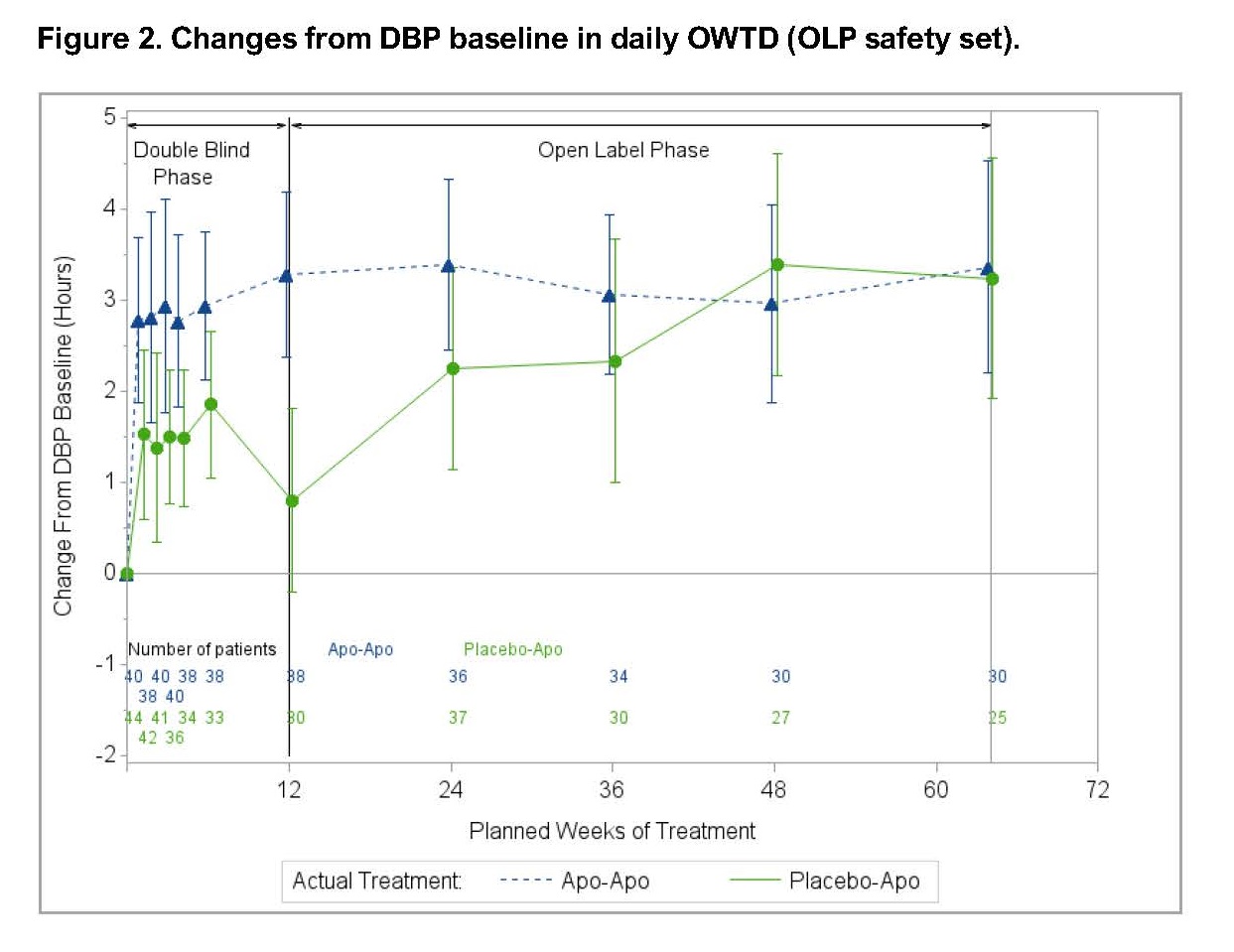
Results: Eighty-four patients entered the OLP (40 previously on APO; 44 APO naïve) and 59 patients (70.2%) completed the study (30 previous APO; 29 APO naïve). The safety profile of APO was consistent with extensive clinical experience (Table 1). Common treatment-related adverse events (AEs) were mild or moderate infusion site nodules, somnolence and nausea. Fourteen (16.7%) patients discontinued the OLP due to AEs; such AEs involving >1 patient were fatigue (n=2) and infusion site reactions (n=4); other AEs are shown in Table 1. There were no significant changes in QT interval, mild reductions in standing systolic blood pressure (-7 mmHg from baseline), slight increases in daytime sleepiness (Epworth Sleepiness Scale: +3.13 points from baseline; within normal range) and no changes in QUIP (Questionnaire for Impulsive–Compulsive Disorders in PD) or C-SSRS (Columbia Suicide Severity Rating Scale) scores. Reduction in daily OFF time (Figure 1) and improvement in ON time without troublesome dyskinesia (OWTD) (Figure 2) were maintained over 64 weeks. Pooled data for Week 64 (n=55) showed a mean (±SD) change from DBP baseline in daily OFF time of -3.66 (2.72) hours and in daily OWTD of 3.31 (3.12) hours. Mean daily levodopa dose decreased from DBP baseline by >260 mg while levodopa-equivalent dose decreased by >500 mg.
Conclusion: The 1-year OLP extension of the first randomised study of APO confirms sustained clinical benefit in PD patients with persistent motor fluctuations. Long-term safety and efficacy were consistent with previous experience from extensive clinical use and observational studies.
References: 1. Katzenschlager R, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018 Sep;17(9):749-759.
To cite this abstract in AMA style:
R. Katzenschlager, W. Poewe, O. Rascol, C. Trenkwalder, G. Deuschl, K. Chaudhuri, T. Henriksen, T. van Laar, D. Lockhart, A. Lees. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease (PD) patients with persistent motor fluctuations: results of the 1-year open-label phase of the TOLEDO study [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-of-apomorphine-infusion-in-parkinsons-disease-pd-patients-with-persistent-motor-fluctuations-results-of-the-1-year-open-label-phase-of-the-toledo-study/. Accessed December 23, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-safety-and-efficacy-of-apomorphine-infusion-in-parkinsons-disease-pd-patients-with-persistent-motor-fluctuations-results-of-the-1-year-open-label-phase-of-the-toledo-study/