Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: SynAgile has developed the DopaFuse Delivery System to noninvasively and continuously administer oral LD/CD into the mouths of patients with PD. The System is intended to reduce fluctuation in plasma levodopa levels and the associated motor symptoms. The objective of this study is to test whether the System can be used safely by the intended users in the a planned Phase 2 clinical trial.
Background: DopaFuse is the first of a new class of drug delivery devices that reside in the mouth and that non-invasively and continuously deliver drugs to patients at a constant rate for absorption via the gastro-intestinal and/or buccal routes. The System consists of a reusable custom dental retainer, its case, and a pre-filled, single-use drug container, shown in Figures 1-3.[figure1][figure2][figure3] SynAgile conducted a single proof-of-concept study in 2015 to test the hypothesis that continuous oral delivery of LD/CD results in improved LD pharmacokinetics and reduced Off time. The study found significantly less variability in plasma LD concentration and reduced Off with continuous versus intermittent oral levodopa delivery.[1]
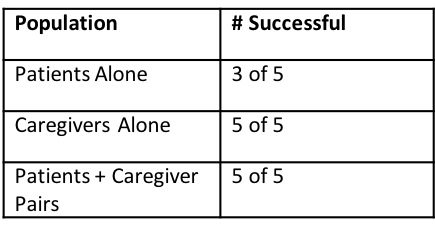
Method: Five patient-participant pairs were invited to participate in this formative human factors study. Each pair consisted of one patient with advanced PD along with their adult caregiver. Pairs attended a single training session followed by a one-hour decay period before testing. Testing was conducted with the patient participant alone, with the caregiver participant alone, and the patient-caregiver pair together. During each session, the participant was asked to set up the system for use, simulate use on a manikin, and store the System. Success was defined as ability to complete the tasks safely without describing or encountering difficulty. During each session, the participant was asked to set up the system for use, simulate use on a manikin, and store the System. Success was defined as ability to complete the tasks safely without describing or encountering difficulty.
Results: [figure4] At no time did any patient or caregiver perform a task in a manner likely to lead to an adverse event during the clinical trial.
Conclusion: This study suggests that patients with advanced PD and their caregivers will be able to safely use the DopaFuse Delivery System in the upcoming Phase 2 clinical trial.
References: [1]Warren Olanow et al. (2019), Continuous versus intermittent oral administration of levodopa in Parkinson’s disease patients with motor fluctuations: A pharmacokinetics, safety, and efficacy study. Mov Disord. doi:10.1002/mds.27610
To cite this abstract in AMA style:
J. Harmon, S. Japp, C. Long, J. Spiridigliozzi, A. Heller, B. Heller, C. King, P. Plante, R. Draper, T. Lau, E. Heller. A Human Factors Study of the DopaFuse® Delivery System [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/a-human-factors-study-of-the-dopafuse-delivery-system/. Accessed April 2, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-human-factors-study-of-the-dopafuse-delivery-system/