Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To characterize the efficacy and safety of zonisamide (ZNS) in patients with dementia with Lewy bodies (DLB) with parkinsonism, a pooled analysis of two trials was performed.
Background: We have conducted exploratory phase 2 [1] and confirmatory phase 3 [2] trials (multicenter, randomized, double-blind, parallel-group, placebo-controlled trials) and reported that ZNS improves parkinsonism in DLB.
Method: In efficacy analysis (n=498), ZNS (25 or 50 mg) was compared with placebo in terms of (1) changes from baseline in UPDRS part III total and subscale (i.e., tremor [UPDRS items 20, 21], rigidity [UPDRS item 22], bradykinesia [UPDRS items 23, 24, 25, 26, 31], and postural instability/gait disturbance [PIGD, UPDRS items 29, 30]) scores at week (W) 12; (2) the responder (defined as >=10% improvement in UPDRS part III total score) proportion at W4, W8, W12 and W12 (last observation carried forward [LOCF]); and (3) changes from baseline in MMSE and NPI-10 total scores at W12. In safety analysis (n=508), ZNS was compared with placebo in terms of the incidence proportions of adverse events.
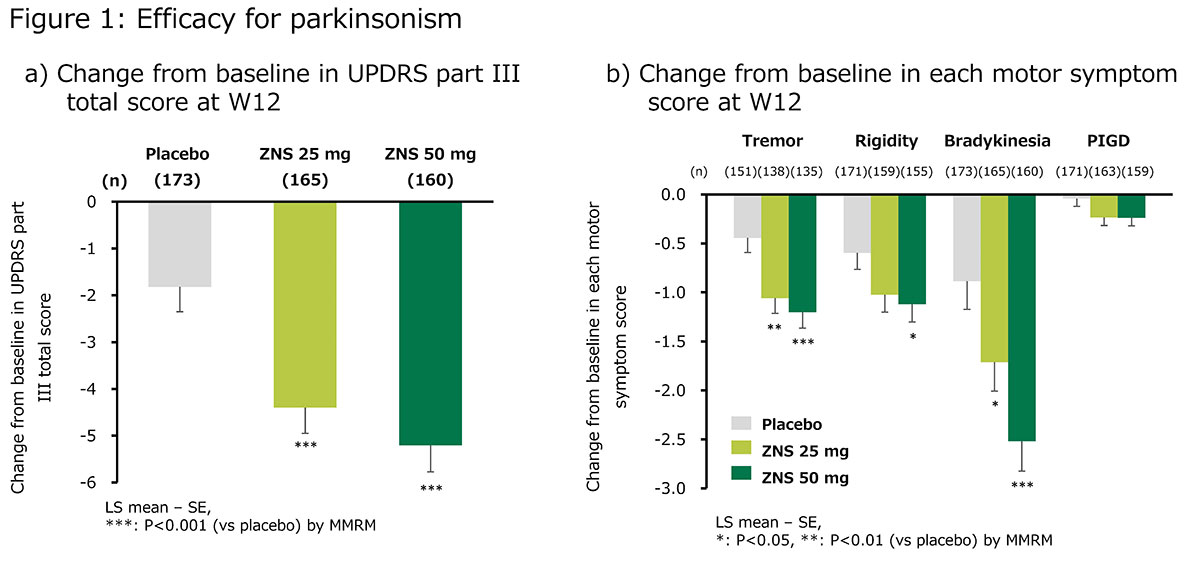
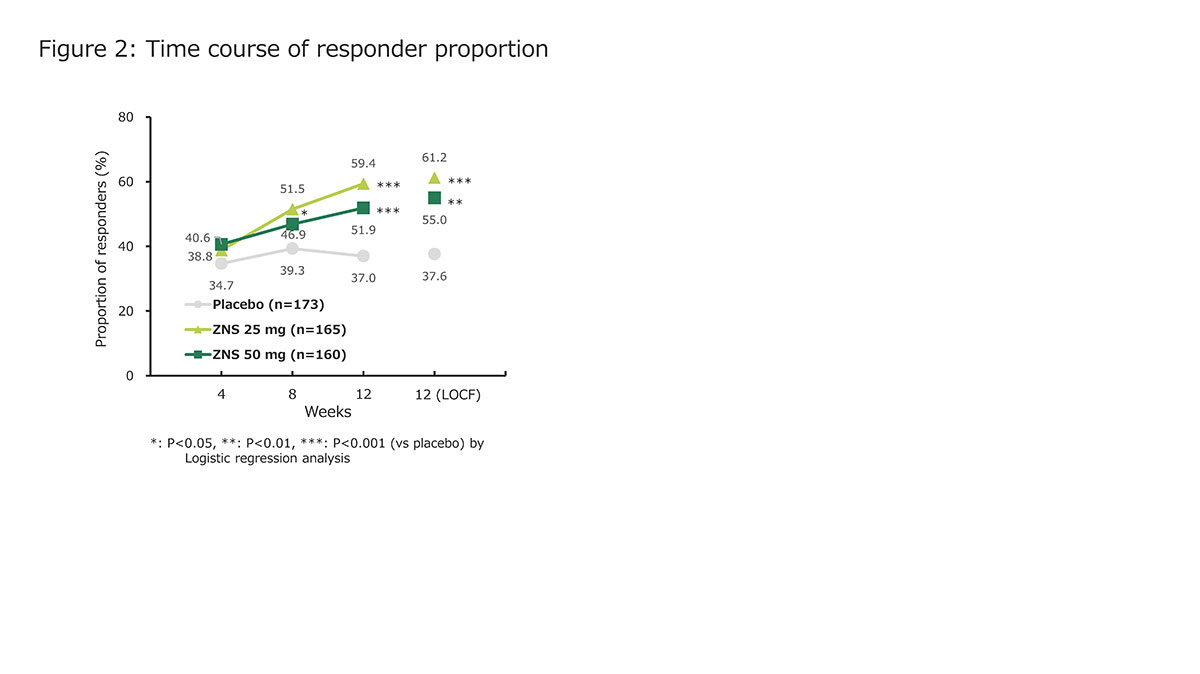
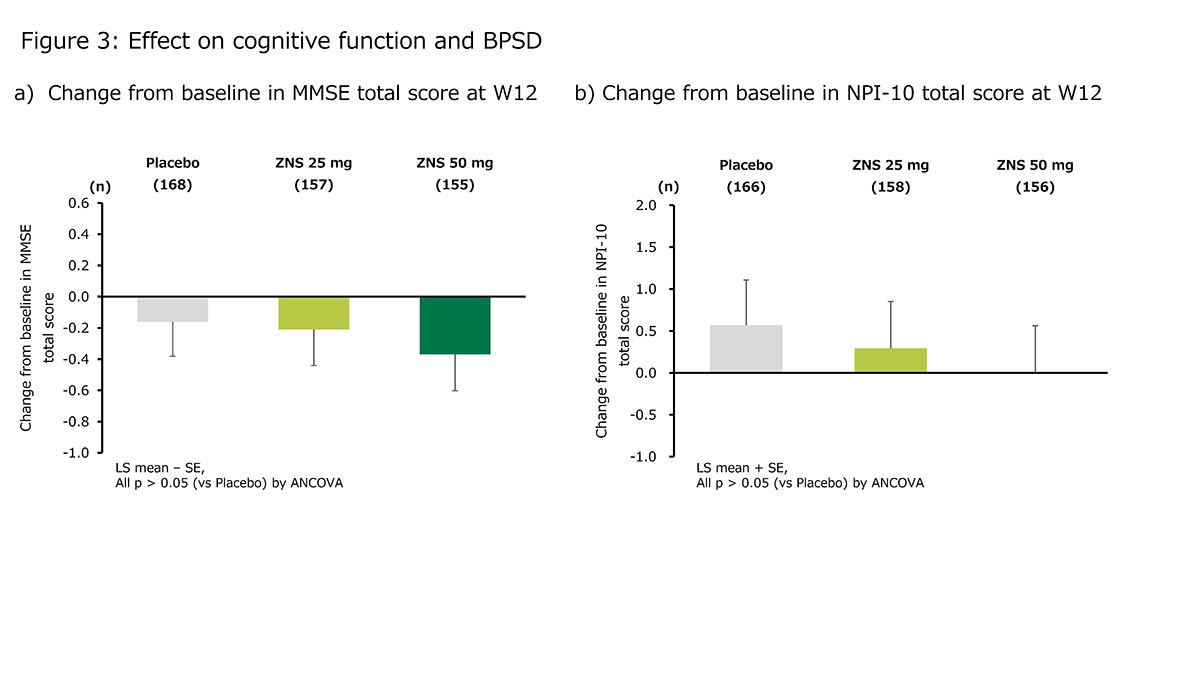
Results: ZNS significantly reduced UPDRS part III total, tremor, rigidity (on ZNS 50 mg only), and bradykinesia scores [figure 1]. The responder proportions at W12 (LOCF) were 61.2%, 55.0%, and 37.6% for ZNS 25 mg, ZNS 50 mg, and placebo, respectively [figure 2]. The changes in MMSE and NPI-10 total scores at W12 did not differ between ZNS (25 or 50 mg) and placebo [figure 3]. The incidence proportion of adverse events did not significantly differ between ZNS and placebo, except for somnolence on ZNS 50 mg.
Conclusion: The analysis indicated that ZNS is effective for parkinsonism in DLB, particularly bradykinesia, tremor and rigidity, and is well tolerated.
References: [1] Murata M, Odawara T, Hasegawa K, et al. Adjunct zonisamide to levodopa for DLB parkinsonism: a randomized, double‐blind phase 2 study. Neurology. 2018; 90: e664-e672. [2] Murata M, Odawara T, Hasegawa K, et al. Zonisamide improves parkinsonism in DLB patients: A randomized phase 3 trial. Mov Disord. 2018; 33 (Suppl. 2).
To cite this abstract in AMA style:
J. Goldman, K. Hasegawa, T. Odawara, K. Kochi, H. Maruyama, O. Konishi, S. Toya, K. Kosaka, M. Murata, I. Mckeith. Efficacy and safety of zonisamide in patients with dementia with Lewy bodies with parkinsonism: pooled analysis of phase 2 and 3 trials [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-zonisamide-in-patients-with-dementia-with-lewy-bodies-with-parkinsonism-pooled-analysis-of-phase-2-and-3-trials/. Accessed January 1, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-zonisamide-in-patients-with-dementia-with-lewy-bodies-with-parkinsonism-pooled-analysis-of-phase-2-and-3-trials/