Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To evaluate the efficacy of PRM in sleep disorders of patients with PD was investigated.
Background: Sleep disorders are highly prevalent in patients with Parkinson’s disease (PD), affecting 88%–98% of patients. Prolonged-release melatonin (PRM, 2 mg; Circadin®), which was approved in Europe in 2007 and in South Korea in July 2014, is widely used to treat sleep disorders. There has been no randomized control trial to evaluate the effectiveness of PRM in PD patients has not been conducted to date.
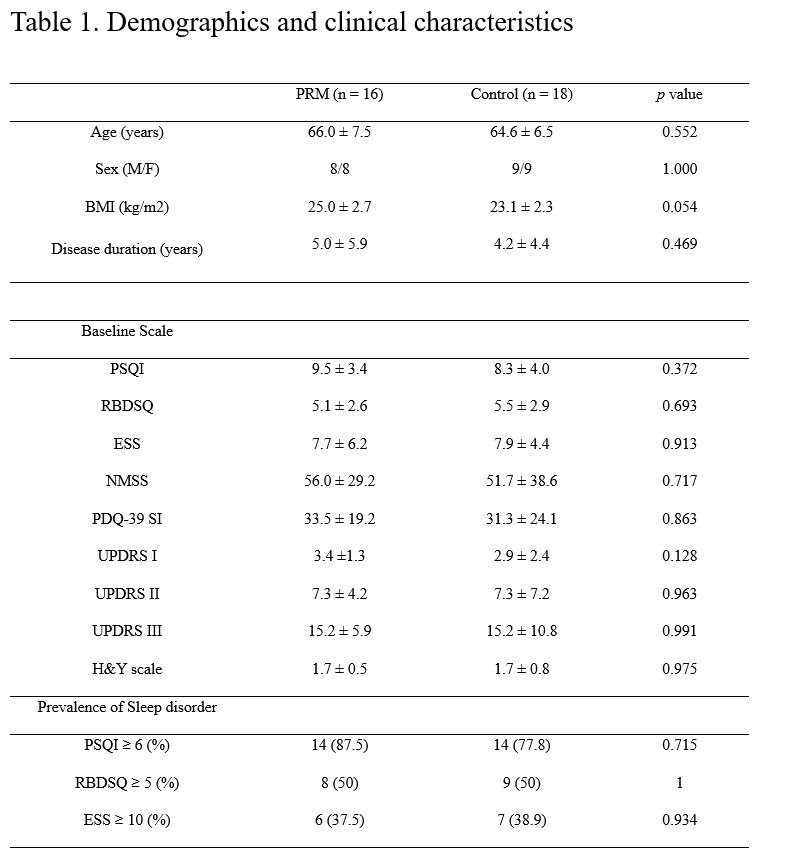
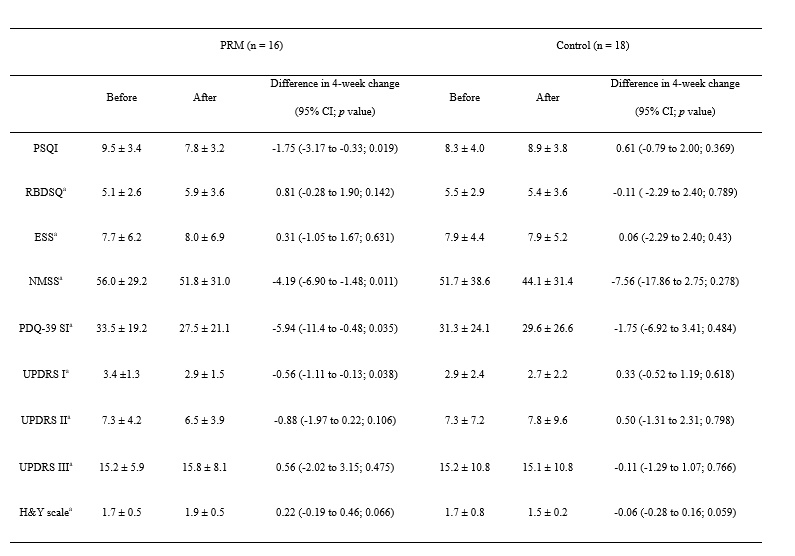
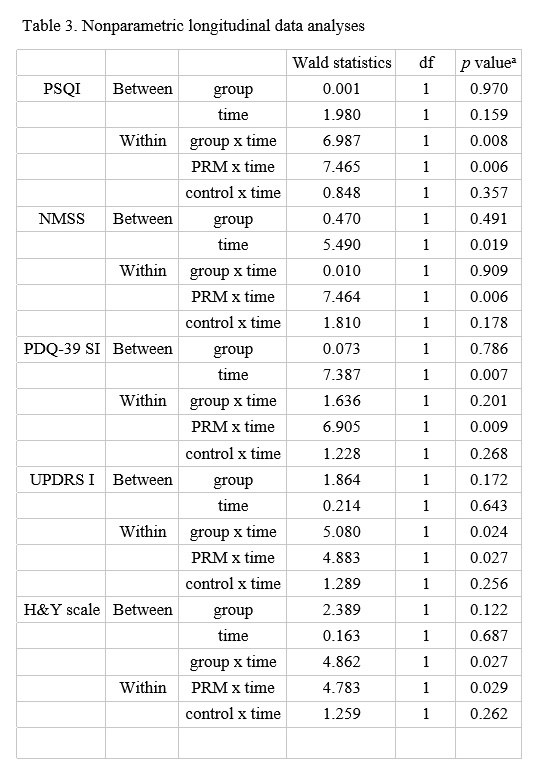
Method: The study was a randomized, double-blind, placebo-controlled, multi-center trial. PD patients who had at least one of the following sleep disorders were included: (1) insomnia; (2) sleep fragmentation; (3) rapid eye movement (REM) sleep behavior disorder (RBD); or (4) excessive daytime sleepiness (EDS). Patients were assessed using the Pittsburgh Sleep Quality Index (PSQI), RBD screening questionnaire (RBDSQ), Epworth Sleepiness Scale (ESS), Non-Motor Symptoms Scale (NMSS), Parkinson’s Disease Quality of Life-39 (PDQ-39), Unified Parkinson’s Disease Rating Scale (UPDRS) I, II, III and Hoehn & Yahr (H&Y) scale at the beginning of the study and after 4 weeks of treatment. Partial correlation analysis was performed to investigate the relationship between PSQI score and other scales.
Results: Sixteen patients were randomized to a PRM group and 18 to a placebo group. PSQI (adjusted p = 0.041), NMSS (adjusted p = 0.030) and PDQ-39 (adjusted p = 0.034) of the PRM group were decreased in the changes over time between groups. Partial correlation analysis showed that improved PSQI score was correlated with lower NMSS score (r = 0.596, p = 0.032) and PDQ-39 (r = 0.582, p = 0.037).
Conclusion: PRM is an effective treatment for sleep disorders in patients with PD and beneficial effects on sleep quality are associated with improved non-motor symptoms (NMS) and quality of life (QoL) in PD patients.
To cite this abstract in AMA style:
JH. Ahn, M. Kim, JK. Mun, J. Youn, S. Park, W. Jang, J. Park, E. Oh, JW. Cho, JS. Kim. Prolonged-release Melatonin for Sleep Disorders in Parkinson’s Disease: A Randomized trial [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/prolonged-release-melatonin-for-sleep-disorders-in-parkinsons-disease-a-randomized-trial/. Accessed January 22, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/prolonged-release-melatonin-for-sleep-disorders-in-parkinsons-disease-a-randomized-trial/