Session Information
Date: Monday, September 23, 2019
Session Title: Huntington’s Disease
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Evaluate the change from baseline at week 52 in Q-Motor (Quantitative Motor) measures, exploratory, standardized, and rater-independent outcomes in the LEGATO-HD study.
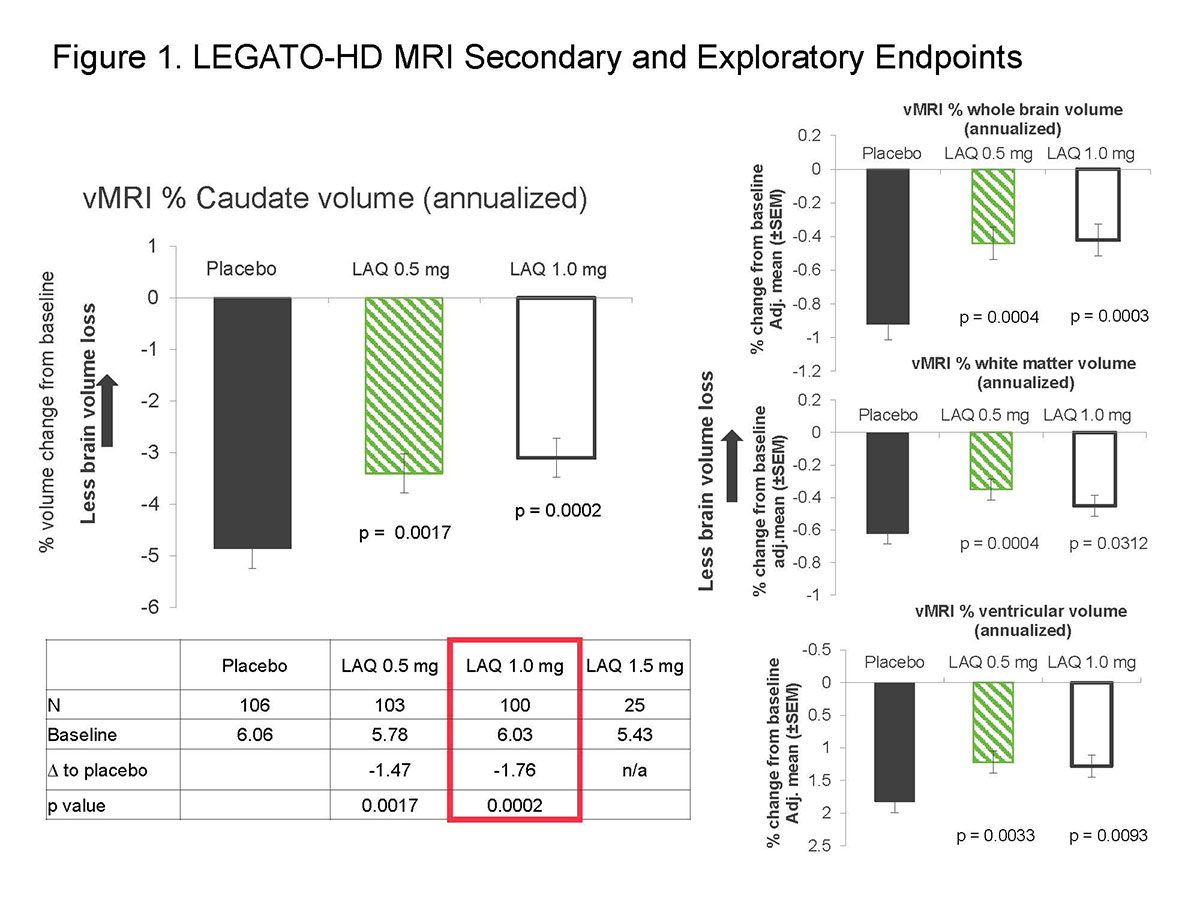
Background: LEGATO-HD assessed laquinimod as treatment for Huntington disease (HD). While the primary endpoint UHDRS-TMS, a categorical rater-dependent scale assessing motor symptoms, did not show changes, the secondary endpoint of benefit in change from baseline in caudate volume was met.
Method: Q-Motor assessments performed at baseline and weeks 4, 13, 26 and 52 included digitomotography (index finger speeded tapping), dysdiadochomotography (pronation/supination hand tapping), manumotography and choreomotography (grip force and chorea analysis), and pedomotography (foot speeded tapping). Data from 48 sites in 10 countries was transferred online for centralized quality control and automated blinded analysis.
Results: Q-Motor data was collected from 317 patients in the placebo, laquinimod 0.5 mg and 1.0 mg treatment arms. Speeded finger tapping (digitomotography) measures (e.g. frequency, duration and variability of inter-onset intervals, inter-peak-intervals, and inter-tap-intervals) demonstrated improvements with nominal statistical significance (p < 0.05) at 0.5 mg, and generally positive trends at 1 mg and is consistent with the reported improvements seen in MRI assessment of brain volume changes. Analysis of pronation/supination (dysdiadochomotography) and foot speeded tapping (pedomotography) in similar parameters to speeded finger tapping showed some positive trends. No relevant changes were seen in grip-force (manumotography) and chorea (choreomotography, i.e., position-index and orientation-index). All measures showed a general worsening in the placebo group.
Conclusion: Q-Motor revealed nominally significant improvements in several tapping measures in the 0.5mg group and positive trends in several parameters for 1 mg group, compared to placebo. Similar to previous studies all Q-Motor measures worsened in the placebo group. The results of the Q motor assessment must be viewed cautiously as corrections for multiplicity were not performed on these analyses, however these observations suggest a central beneficial effect of laquinimod in LEGATO-HD of unknown clinical significance and support a biological relevance of the imaging changes observed.
To cite this abstract in AMA style:
R. Reilmann, M. Gordon, R. Schubert, K. Anderson, A. Feigin, S. Tabrizi, B. Leavitt, J. Stout, P. Piccini, B. Borowsky, G. Rynkowski, R. Volkinshtein, J. Savola, M. Hayden. Quantitative Motor (Q-Motor) Assessments Suggest a Beneficial Central Effect of Laquinimod in a Phase II Study in Huntington Disease (LEGATO-HD) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/quantitative-motor-q-motor-assessments-suggest-a-beneficial-central-effect-of-laquinimod-in-a-phase-ii-study-in-huntington-disease-legato-hd/. Accessed March 31, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/quantitative-motor-q-motor-assessments-suggest-a-beneficial-central-effect-of-laquinimod-in-a-phase-ii-study-in-huntington-disease-legato-hd/