Session Information
Date: Monday, October 8, 2018
Session Title: Parkinson's Disease: Pathophysiology
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
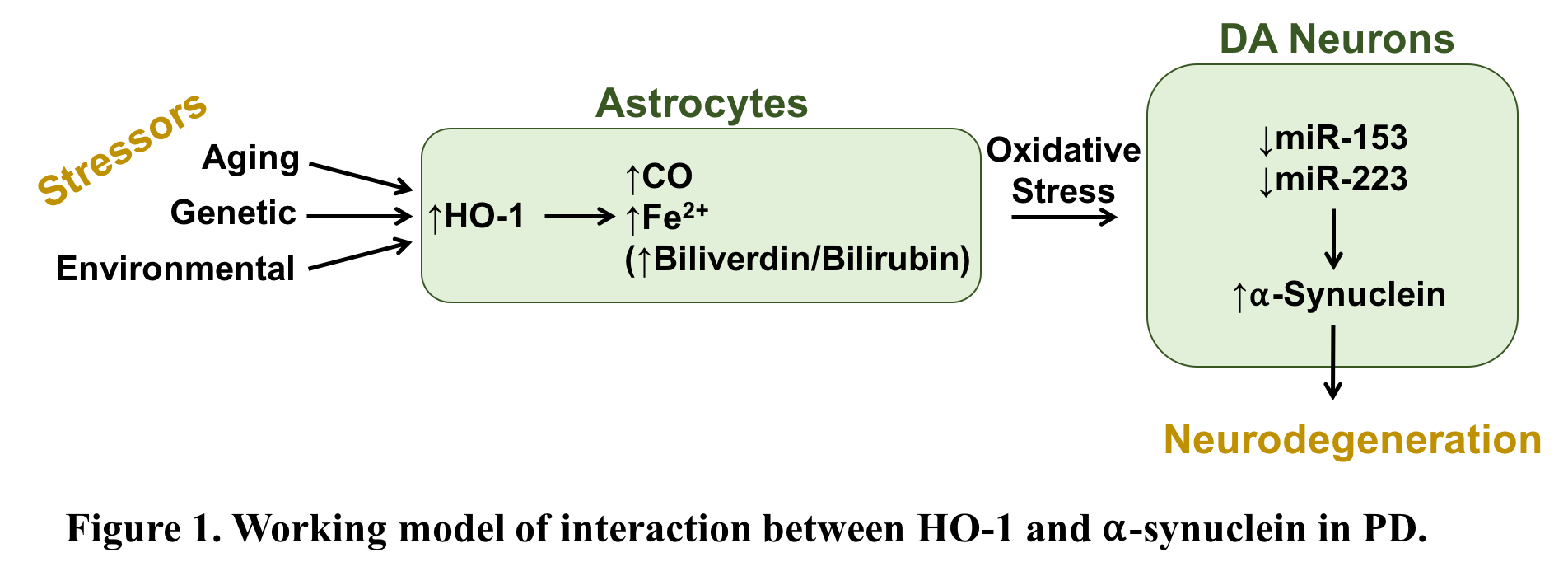
Objective: To determine whether astroglial HO-1 transduces environmental and endogenous stressors into patterns of neural damage which promote the toxicity of neuronal alpha-synuclein.
Background: The product of the stressor-inducible HMOX1 gene, heme oxygenase-1 (HO-1) is highly overexpressed in astrocytes of the substantia nigra in patients with idiopathic Parkinson’s disease (PD) [1]. There is considerable evidence implicating HO-1 in the pathogenesis of PD, and overexpression of astroglial HMOX1 in vitro to levels seen in post-mortem PD brain promotes pathological iron deposition, oxidative stress, mitochondrial damage and macroautophagy characteristic of the human disorder. We recently engineered conditional GFAP.HMOX1 transgenic mice that selectively overexpress human HO-1 in astrocytes [2]. Transgene expression in these mice between 8.5 and 19 months of age results in a parkinsonian phenotype characterized by oxidative stress; basal ganglia siderosis; mitochondrial damage; nigrostriatal hypodopaminergia associated with locomotor incoordination and stereotypy; and overproduction of alpha-synuclein mRNA and protein [2,3]. Alpha-synuclein is a key player in PD pathogenesis and a major constituent of hallmark Lewy pathology. While precise mechanisms of abnormal alpha-synuclein aggregation remain disputed, there is fair consensus implicating oxidative reactions in this process [4,5].
Methods: Primary astrocytes and neurons were isolated from homozygous GFAP.HMOX1 or WT matings on postnatal day 1 [2]. RT-qPCR was used to measure gene expression levels and Western blots were used to measure protein levels [2,3].
Results: Primary WT neurons co-cultured with GFAP.HMOX1 astrocytes exhibit enhanced protein oxidation, mitophagy, and apoptosis, aberrant expression of genes regulating the dopaminergic phenotype and imbalance in genes regulating mitochondrial biogenesis. The latter abnormalities were abrogated by siRNA knock-down of alpha-synuclein, implicating alpha-synuclein as a key mediator of HO-1’s neurodystrophic effects. We also identified two microRNAs (miRNA) that negatively regulate alpha-synuclein in GFAP.HMOX1 mice, namely miR-153 and miR-223.
Conclusions: Taken together, HO-1 downregulates miR-153 and miR-223 which in turn upregulates alpha-synuclein in the GFAP.HMOX1 mouse brain (Fig.1). These results highlight the importance of alpha-synuclein as a therapeutic target, potentially via miR-153 or miR-223, in the treatment of PD.
References: 1. Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp Neurol. 1998;150(1):60-68. 2. Song W, Cressatti M, Zukor H, Liberman A, Galindez C, Schipper HM. Parkinsonian features in aging GFAP.HMOX1 transgenic mice overexpressing human HO-1 in the astroglial compartment. Neurobiol Aging. 2017;58:163-179. 3. Lin SH, Song W, Cressatti M, Zukor H, Wang E, Schipper HM. Heme oxygenase-1 modulates microRNA expression in cultured astroglia: implications for chronic brain disorders. Glia. 2015;63(7):1270-1284. 4. Deas E, Cremades N, Angelova PR, et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid Redox Signal. 2016;24(7):376-391. 5. Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2(7):492-501.
To cite this abstract in AMA style:
M. Cressatti, W. Song, A. Turk, C. Galindez, H. Schipper. Effects of Glial Heme Oxygenase-1 on Neuronal Alpha-Synuclein in the GFAP.HMOX1 Mouse Model of Parkinson’s Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/effects-of-glial-heme-oxygenase-1-on-neuronal-alpha-synuclein-in-the-gfap-hmox1-mouse-model-of-parkinsons-disease/. Accessed April 1, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effects-of-glial-heme-oxygenase-1-on-neuronal-alpha-synuclein-in-the-gfap-hmox1-mouse-model-of-parkinsons-disease/