Session Information
Date: Monday, October 8, 2018
Session Title: Parkinson's Disease: Non-Motor Symptoms
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: SIAXI (NCT02091739), a pivotal double-blind, randomised, placebo-controlled study with a 48-week extension period (EP), assessed the efficacy and safety of incobotulinumtoxinA 75 U or 100 U in patients with sialorrhea due to Parkinson’s disease (PD), atypical parkinsonism, stroke or traumatic brain injury. Data from the placebo-controlled main period (MP) are reported elsewhere. Here, we present data from the complete study.
Background: Current treatment for chronic, troublesome sialorrhea is based on multidisciplinary management of drooling, with no approved pharmacological agents.
Methods: In the MP patients were randomised (2:2:1) to incobotulinumtoxinA 75 or 100 U (n=74 each), or placebo (n=36) in a single injection cycle (IC). At completion, eligible patients entered the EP and received three further incobotulinumtoxinA ICs (each 16±2 weeks) of 75 U or 100 U. Placebo recipients were randomised 1:1 to receive either 75 U or 100 U incobotulinumtoxinA in the EP. Outcomes included: unstimulated salivary flow rate (uSFR), patients’ Global Impression of Change Scale (GICS), Drooling Severity and Frequency Scale (DSFS), modified Radboud Oral Motor Inventory for Parkinson’s Disease (mROMP) (Kalf JG, Arch Phys Med Rehabil 2011), EuroQoL 5-dimensions visual analogue scale (EQ-5D VAS) and adverse events (AEs).
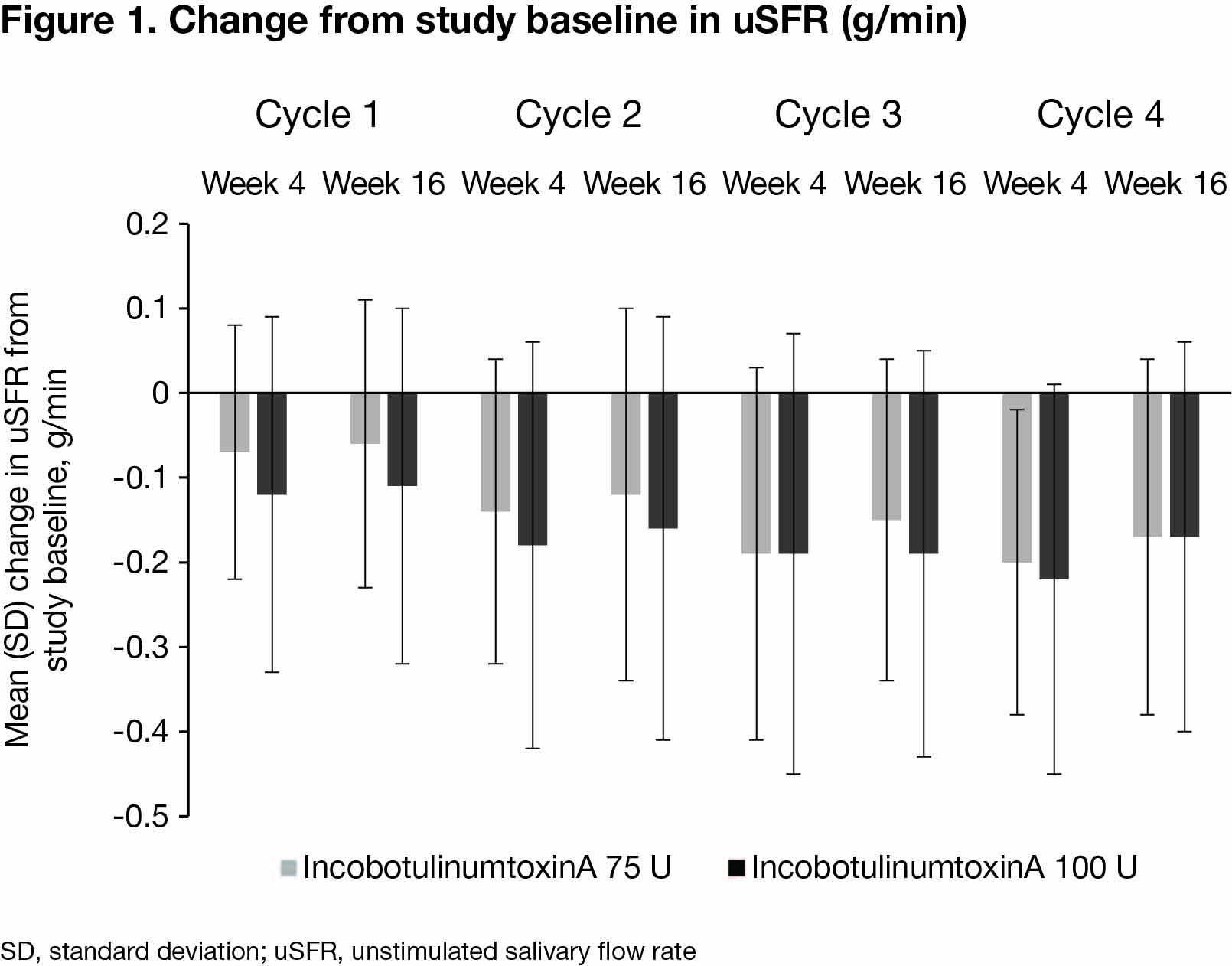
Results: Of 184 patients randomised, 173 (mean [SD] age 65.2 [11.4] years; 71.7% male; 71.1% with sialorrhea due to PD) completed the MP and entered the EP. Among 68 and 72 patients treated with 75 U or 100 U incobotulinumtoxinA in all 4 ICs, mean (SD) uSFR decreased continuously from study baseline with repeated ICs [figure 1]. Patients’ GICS showed improvements at all visits, with mean (SD) improvements of 1.29 (1.18) and 1.41 (1.18) at IC4 end. DSFS, mROMP drooling scores and EQ-5D VAS also improved from study baseline to 4 weeks post-injection in each IC. mROMP speech and swallowing symptom scores remained stable. The most common treatment-related AEs were dry mouth (4.4% and 11.1%) and dysphagia (1.5% and 4.2%). Serious treatment-related AEs were speech disorder (1/68 [1.5%] incobotulinumtoxinA 75 U recipient) and dysphagia (1/72 [1.4%] 100 U recipient).
Conclusions: These data support the long-term efficacy and safety of repeated incobotulinumtoxinA treatment for sialorrhea of neurological origin.
References: Kalf JG, Borm GF, de Swart BJ, Bloem BR, Zwarts MJ, Munneke M. Reproducibility and validity of patient-rated assessment of speech, swallowing, and saliva control in Parkinson’s disease. Arch Phys Med Rehabil. 2011;92(7):1152-8.
To cite this abstract in AMA style:
F. Pagan, W. Jost, A. Friedman, O. Michel, C. Oehlwein, J. Slawek, A. Bogucki, S. Ochudlo, M. Banach, B. Flatau-Baqué, J. Csikós, A. Blitzer. Long-term efficacy and safety of incobotulinumtoxinA treatment for sialorrhea in Parkinson’s disease and other neurologic conditions [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/long-term-efficacy-and-safety-of-incobotulinumtoxina-treatment-for-sialorrhea-in-parkinsons-disease-and-other-neurologic-conditions/. Accessed April 1, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-efficacy-and-safety-of-incobotulinumtoxina-treatment-for-sialorrhea-in-parkinsons-disease-and-other-neurologic-conditions/