Session Information
Date: Monday, October 8, 2018
Session Title: Parkinson's Disease: Genetics
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: The pathogenic mechanism of UCHL1 in PD remains unclear. In this study, we aimed to investigate the role of UCHL1 in the pathogenesis of PD.
Background: Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. The ubiquitin carboxy-terminal hydrolase L1 (UCHL1), a member of deubiquitinating enzymes, is primarily abundant in neurons. Down-regulation and dysfunction of UCHL1 have been detected in the brains of PD patients. In addition, UCHL1 gene mutants are linked to PD.
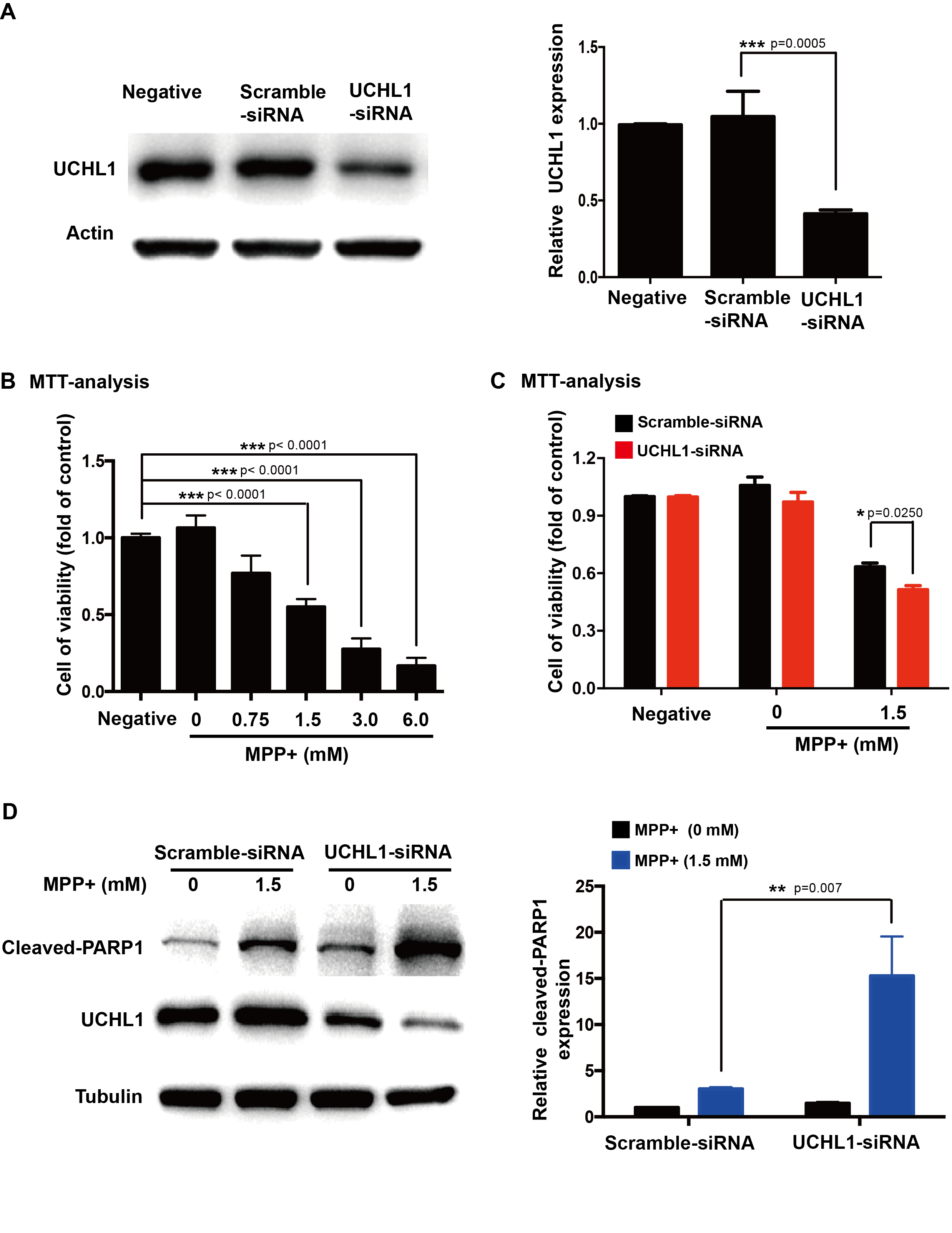
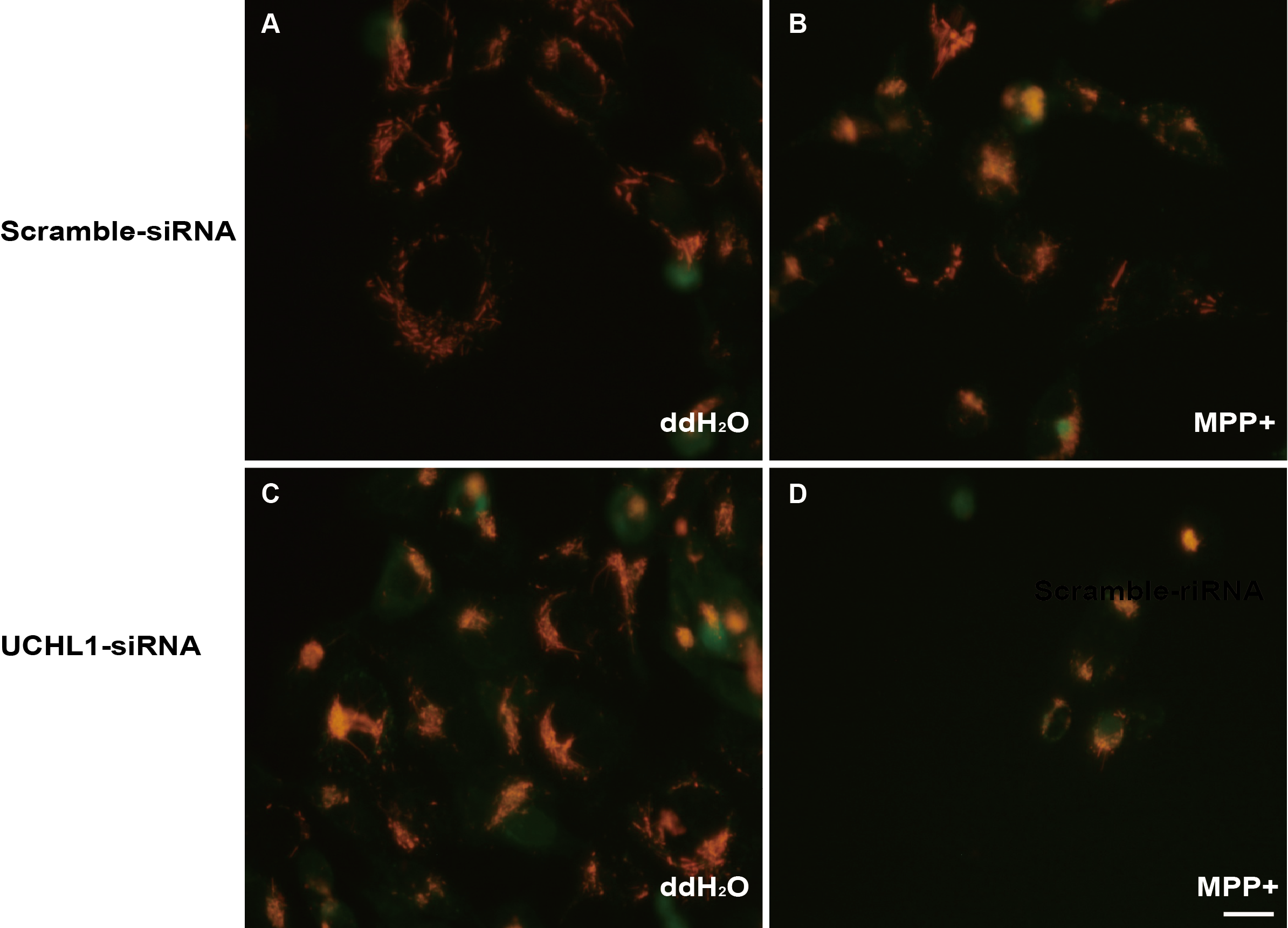
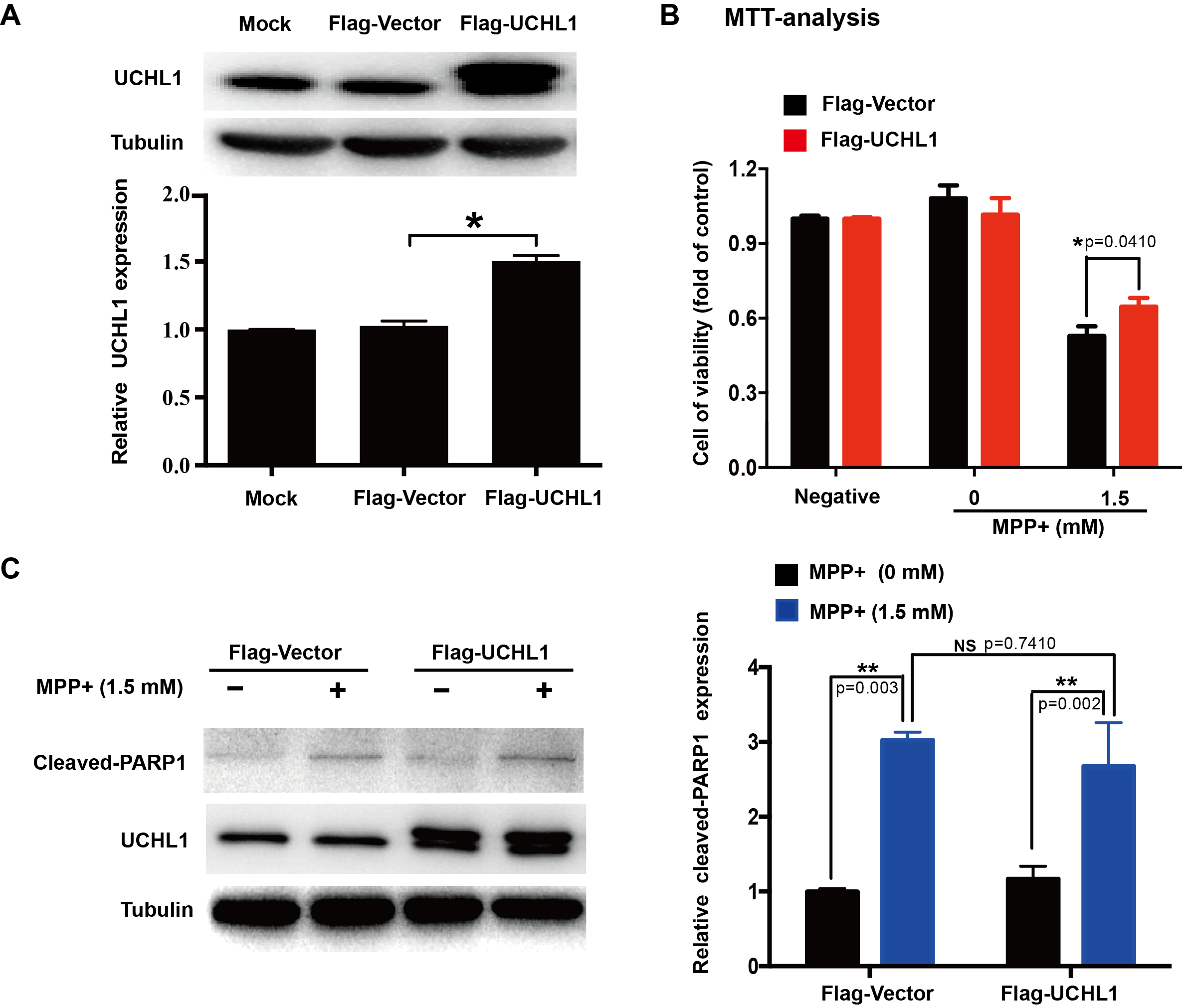
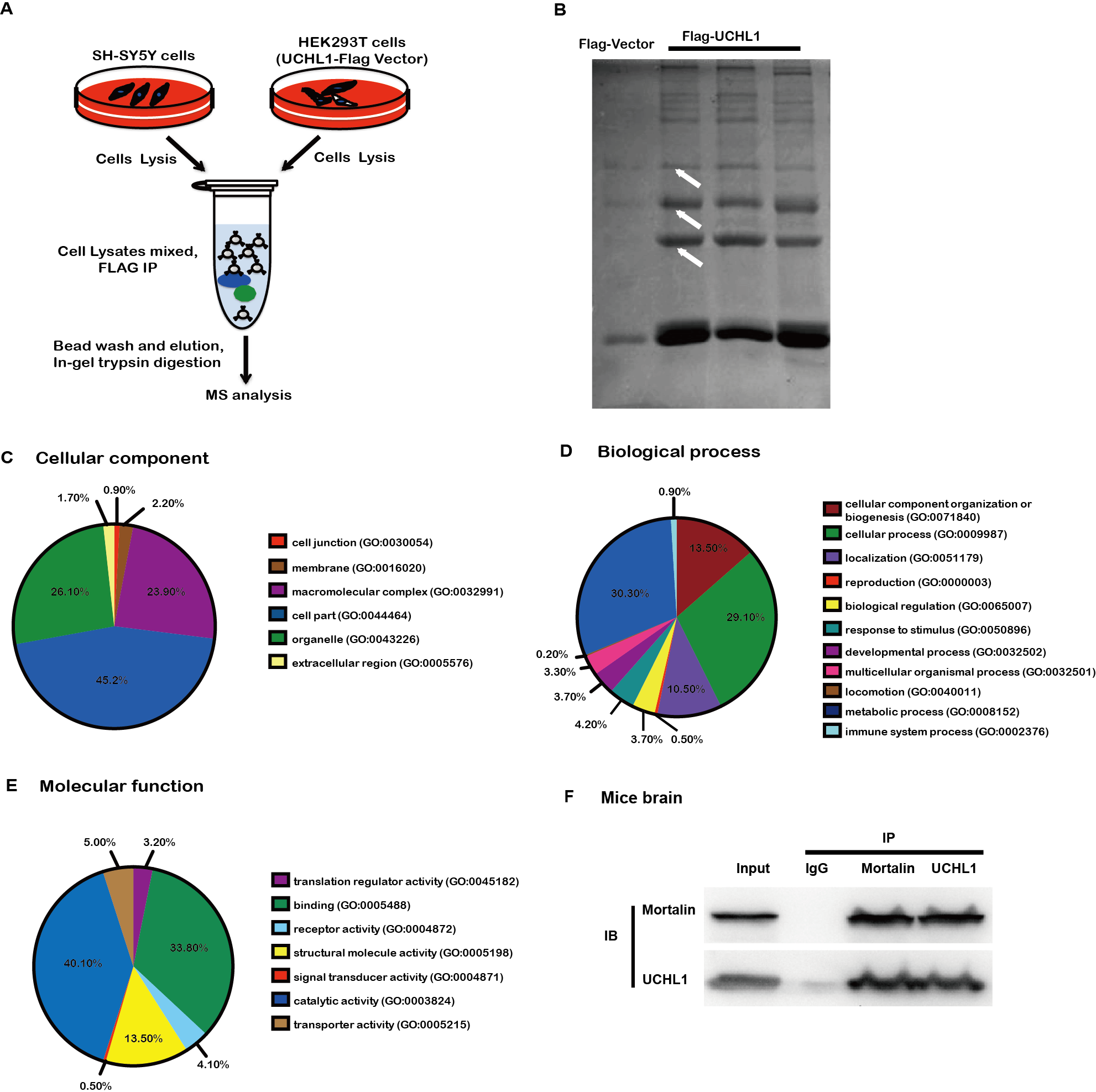
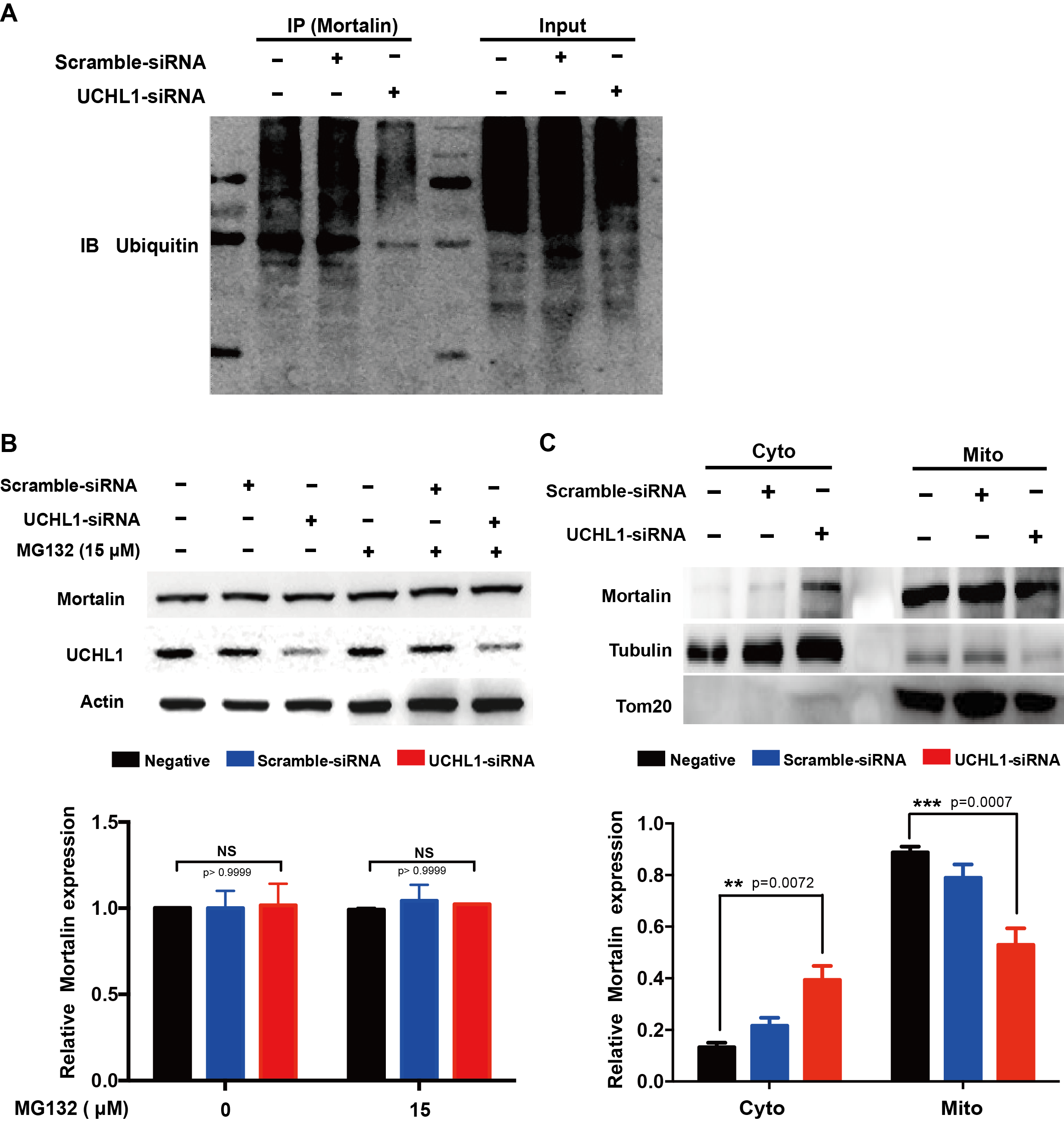
Methods: To study the mechanism of UCHL1 in the pathogenesis of PD, we used MPP+ to reproduce a cellular model of PD in SH-SY5Y cells. The effects of UCHL1 knockdown and overexpression in MPP+-induced cellular model of PD were determined. Immunoprecipitation and mass spectrometric analysis (IP-MS) were used to screen UCHL1 interacting proteins. Among these proteins, we focused on Mortalin. The interaction between UCHL1 and Mortalin was confirmed by Co-immunoprecipitation (Co-IP) in vivo and vitro using mice brain and HEK293T cells lysates. Co-IP was also applied to detect whether UCHL1 affects Mortalin ubiquitination. We used MG132 to block proteasome-dependent degradation and tested whether UCHL1 would influence Mortalin protein level in SH-SY5Y cells. UCHL1-siRNA transfected SH-SY5Y cells were fractionated into cytoplasmic and mitochondrial fractions, followed by western blotting to detect Mortalin localization change.
Results: UCHL1 knockdown enhanced MPP+-induced cytotoxicity in SH-SY5Y cells, as reflected by decreased cell viability, increased apoptosis protein level and declined mitochondrial membrane potential, while UCHL1 overexpression could rescue MPP+-induced cytotoxicity in SH-SY5Y cells. In total, 234 UCHL1 interacting proteins were screened through IP-MS. According to the findings, Mortalin was chosen as a target protein due to its association with PD reported previously. Mortalin co-immunoprecipitated with Mortalin in vivo and vitro was from rat brains and HEK293T cells lysates respectively. Furthermore, UCHL1 ubiquitinated Mortalin as ubiquitin ligase. Instead of affecting the protein level of Mortalin, UCHL1-mediated ubiquitination facilitated the localization of Mortalin to mitochondria.
Conclusions: The present data suggested that UCHL1 could mediate Mortalin to mitochondrial localization by ubiquitinating Mortalin as an ubiquitin ligase.
References: 1. Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin. 1996;14(2):317-35. PubMed PMID: 8827174. 2. Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395(6701):451-2. doi: 10.1038/26652. PubMed PMID: 9774100. 3. Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40(6):1542-7. PubMed PMID: 6343558. 4. Barrachina M, Castano E, Dalfo E, Maes T, Buesa C, Ferrer I. Reduced ubiquitin C-terminal hydrolase-1 expression levels in dementia with Lewy bodies. Neurobiol Dis. 2006;22(2):265-73. doi: 10.1016/j.nbd.2005.11.005. PubMed PMID: 16380264. 5. Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279(13):13256-64. doi: 10.1074/jbc.M314124200. PubMed PMID: 14722078. 6. Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT, Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111(2):209-18. PubMed PMID: 12408865. 7. Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry. 1998;37(10):3358-68. doi: 10.1021/bi972274d. PubMed PMID: 9521656. 8. Meray RK, Lansbury PT, Jr. Reversible monoubiquitination regulates the Parkinson disease-associated ubiquitin hydrolase UCH-L1. J Biol Chem. 2007;282(14):10567-75. doi: 10.1074/jbc.M611153200. PubMed PMID: 17259170. 9. Suong DN, Thao DT, Masamitsu Y, Thuoc TL. Ubiquitin carboxyl hydrolase L1 significance for human diseases. Protein Pept Lett. 2014;21(7):624-30. PubMed PMID: 24702261. 10. Gatt AP, Duncan OF, Attems J, Francis PT, Ballard CG, Bateman JM. Dementia in Parkinson’s disease is associated with enhanced mitochondrial complex I deficiency. Mov Disord. 2016;31(3):352-9. doi: 10.1002/mds.26513. PubMed PMID: 26853899. 11. Michel PP, Hirsch EC, Hunot S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron. 2016;90(4):675-91. doi: 10.1016/j.neuron.2016.03.038. PubMed PMID: 27196972. 12. Hu Q, Wang G. Mitochondrial dysfunction in Parkinson’s disease. Transl Neurodegener.2016 5:142016 Oct 31;5:19. PMID:27822367. 13. Geissler A, Rassow J, Pfanner N, Voos W. Mitochondrial import driving forces: enhanced trapping by matrix Hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol Cell Biol. 2001;21(20):7097-104. doi: 10.1128/MCB.21.20.7097-7104.2001. PubMed PMID: 11564892; PubMed Central PMCID: PMCPMC99885. 14. Kaul SC, Deocaris CC, Wadhwa R. Three faces of mortalin: a housekeeper, guardian and killer. Exp Gerontol. 2007;42(4):263-74. doi: 10.1016/j.exger.2006.10.020. PubMed PMID: 17188442. 15. Shi M, Jin J, Wang Y, Beyer RP, Kitsou E, Albin RL, et al. Mortalin: a protein associated with progression of Parkinson disease? J Neuropathol Exp Neurol. 2008;67(2):117-24. doi: 10.1097/nen.0b013e318163354a. PubMed PMID: 18219256. 16. Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, et al. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics. 2006;5(7):1193-204. doi: 10.1074/mcp.M500382-MCP200. PubMed PMID: 16565515. 17. Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, et al. Dissecting the role of the mitochondrial chaperone mortalin in Parkinson’s disease: functional impact of disease-related variants on mitochondrial homeostasis. Hum Mol Genet. 2010;19(22):4437-52. doi: 10.1093/hmg/ddq370. PubMed PMID: 20817635; PubMed Central PMCID: PMCPMC3298849. 18. De Mena L, Coto E, Sanchez-Ferrero E, Ribacoba R, Guisasola LM, Salvador C, et al. Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J Neural Transm (Vienna). 2009;116(10):1289-93. doi: 10.1007/s00702-009-0273-2. PubMed PMID: 19657588. 19. Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002;7(3):309-16. PubMed PMID: 12482206; PubMed Central PMCID: PMCPMC514830. 20. McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol. 2003;179(1):38-46. PubMed PMID: 12504866. 21. McNaught KS, Olanow CW. Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson’s disease. Ann Neurol. 2003;53 Suppl 3:S73-84; discussion S-6. doi: 10.1002/ana.10512. PubMed PMID: 12666100. 22. Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, Kiyosawa H, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet. 1999;23(1):47-51. doi: 10.1038/12647. PubMed PMID: 10471497. 23. Coulombe J, Gamage P, Gray MT, Zhang M, Tang MY, Woulfe J, et al. Loss of UCHL1 promotes age-related degenerative changes in the enteric nervous system. Front Aging Neurosci. 2014;6:129. doi: 10.3389/fnagi.2014.00129. PubMed PMID: 24994982; PubMed Central PMCID: PMCPMC4063237. 24. Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One. 2009;4(8):e6764. doi: 10.1371/journal.pone.0006764. PubMed PMID: 19707515; PubMed Central PMCID: PMCPMC2729380. 25. Chiasserini D, Tozzi A, de Iure A, Tantucci M, Susta F, Orvietani PL, et al. Mortalin inhibition in experimental Parkinson’s disease. Mov Disord. 2011;26(9):1639-47. doi: 10.1002/mds.23647. PubMed PMID: 21542017. 26. Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, et al. Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells. J Neurochem. 2007;103(5):1989-2003. doi: 10.1111/j.1471-4159.2007.04907.x. PubMed PMID: 17868329. 27. Liu Y, Liu W, Song XD, Zuo J. Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem. 2005;268(1-2):45-51. PubMed PMID: 15724436. 28. Qu M, Zhou Z, Xu S, Chen C, Yu Z, Wang D. Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2011;1368:336-45. doi: 10.1016/j.brainres.2010.10.068. PubMed PMID: 20974113. 29. Hershko A, Ciechanover A, Varshavsky A. Basic Medical Research Award. The ubiquitin system. Nat Med. 2000;6(10):1073-81. doi: 10.1038/80384. PubMed PMID: 11017125. 30. Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem. 2001;276(35):33111-20. doi: 10.1074/jbc.M102755200. PubMed PMID: 11435423. 31. Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159-80. doi: 10.1146/annurev.cellbio.22.010605.093503. PubMed PMID: 16753028. 32. Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6(8):599-609. doi: 10.1038/nrm1700. PubMed PMID: 16064136. 33. Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203-29. doi: 10.1146/annurev-biochem-060310-170328. PubMed PMID: 22524316. 34. Chin LS, Li L. Ubiquitin phosphorylation in Parkinson’s disease: Implications for pathogenesis and treatment.Transl Neurodegener. 2016 Jan 6;5:1. doi: 10.1186/s40035-015-0049-6. PMID:26740872.
To cite this abstract in AMA style:
L. Wu, W. Yang, Y. Tan, J. Ding, S. Chen. Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) -mediated ubiquitination attributed to localization of Mortalin to mitochondria [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/ubiquitin-carboxyl-terminal-hydrolase-1-uchl1-mediated-ubiquitination-attributed-to-localization-of-mortalin-to-mitochondria/. Accessed December 26, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/ubiquitin-carboxyl-terminal-hydrolase-1-uchl1-mediated-ubiquitination-attributed-to-localization-of-mortalin-to-mitochondria/