Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: Design an experiment to assess tremor severity comparable to the current clinical standard using Shimmer3 sensors. Investigate collected data to see if BEST intervention affects tremor.
Background: One of the symptoms associated with Parkinson’s disease (PD) is tremor of a person’s body parts.1 A bio-electro stimulation therapeutic (BEST) device known as the E-tapper developed by ImmuMax has received anecdotal evidence of improving motor symptoms such as resting tremor (RT) in patients with PD.7 In the present pilot-study, the assessment of tremor will be used to investigate the effectiveness of the E-tapper in treating motor and non-motor symptoms of PD. Our project will develop an objective measure of tremor in patients with PD.
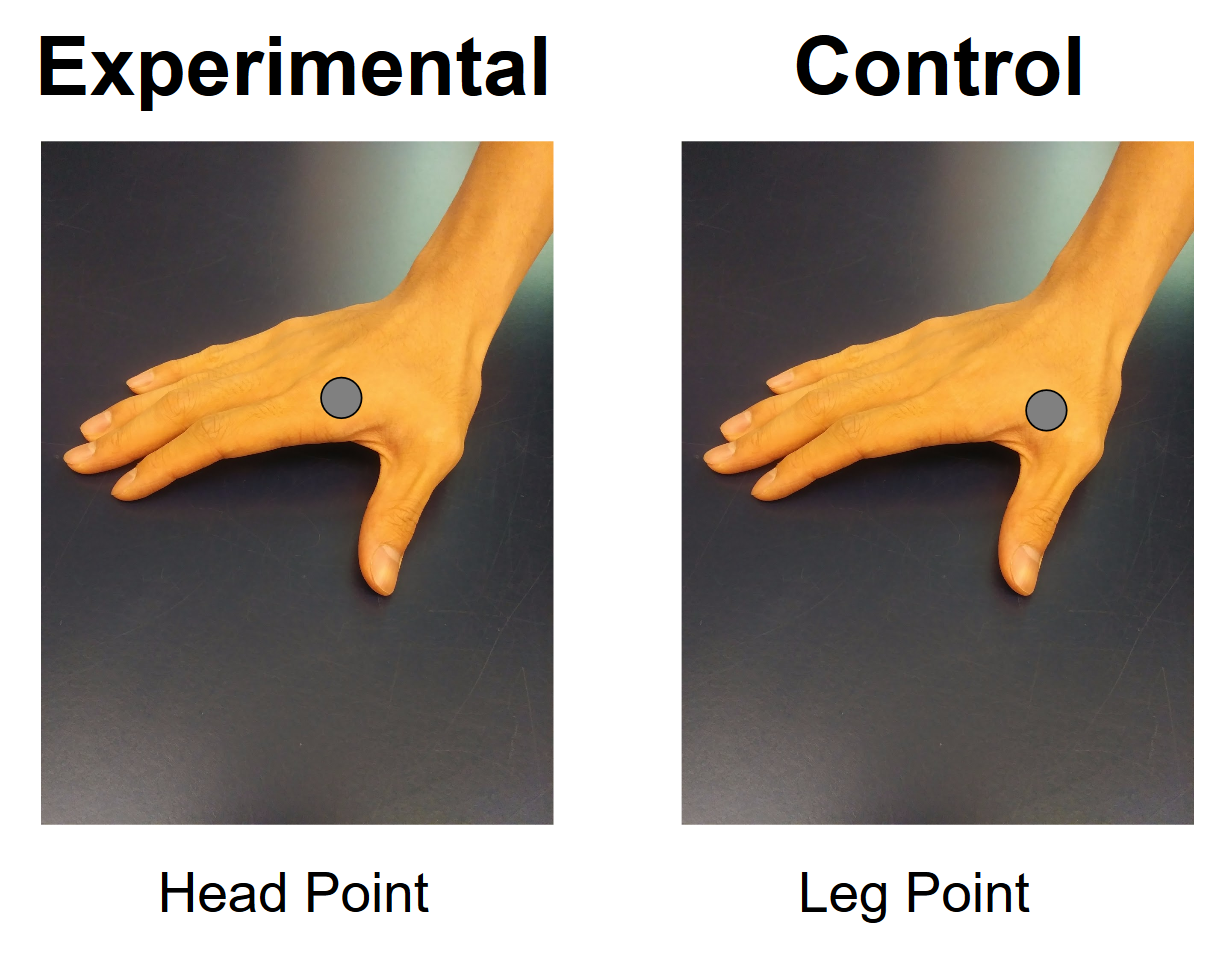
Methods: BEST Intervention. The study has an experimental group receiving BEST at the Head Point (Figure 1A) and a control group receiving BEST at the Leg Point (Figure 1B). Initial RT tremor data is taken and the UPDRS is administered. BEST is administered twice a day at the specified point for 30 minutes on each hand over a course of 10 weeks. After the intervention, the UPDRS was administered again and post-BEST RT data was collected. Developing the machine learning algorithm. Power spectral density analysis was used to identify the frequencies present in the tremor of a single patient. A Naive Bayes Classifier machine learning algorithm was developed to identify PD patients receiving BEST and PD patients receiving the control treatment using non-PD patients as a baseline. With repeated training and optimization, a network based on a collected quantitative data could potentially determine where on the UPDRS scale a PD patient lies.
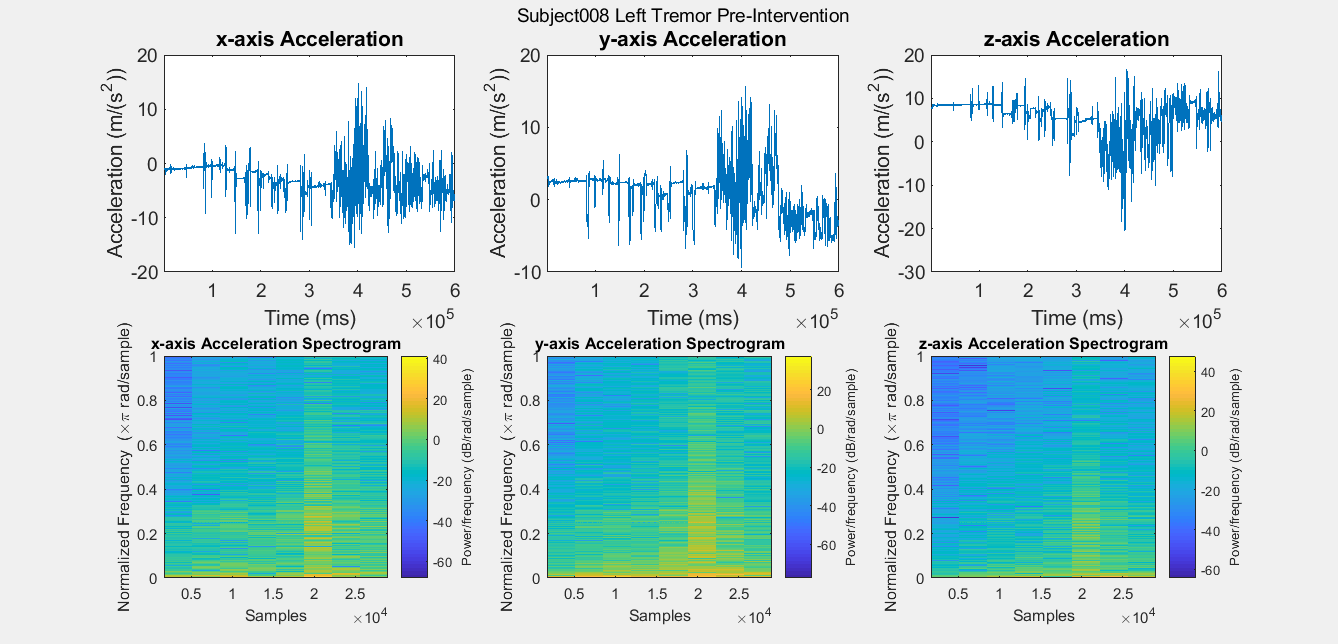
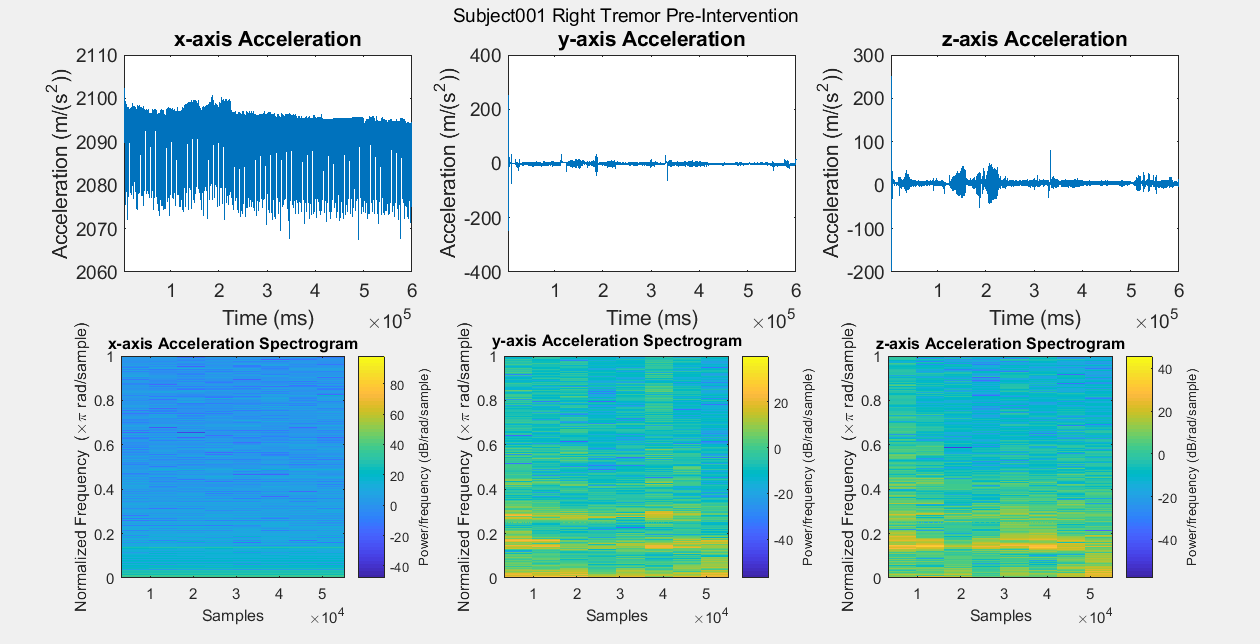
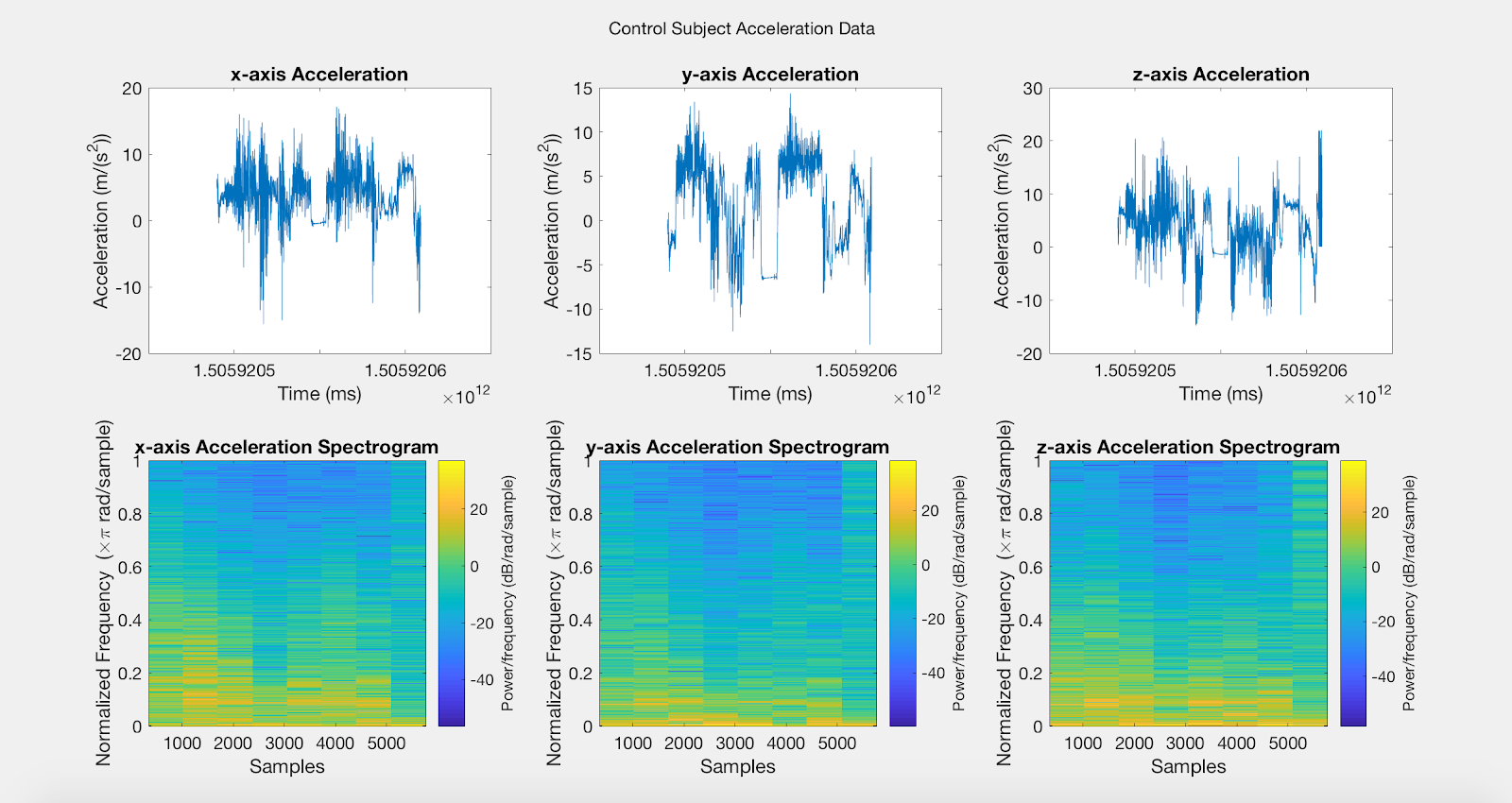
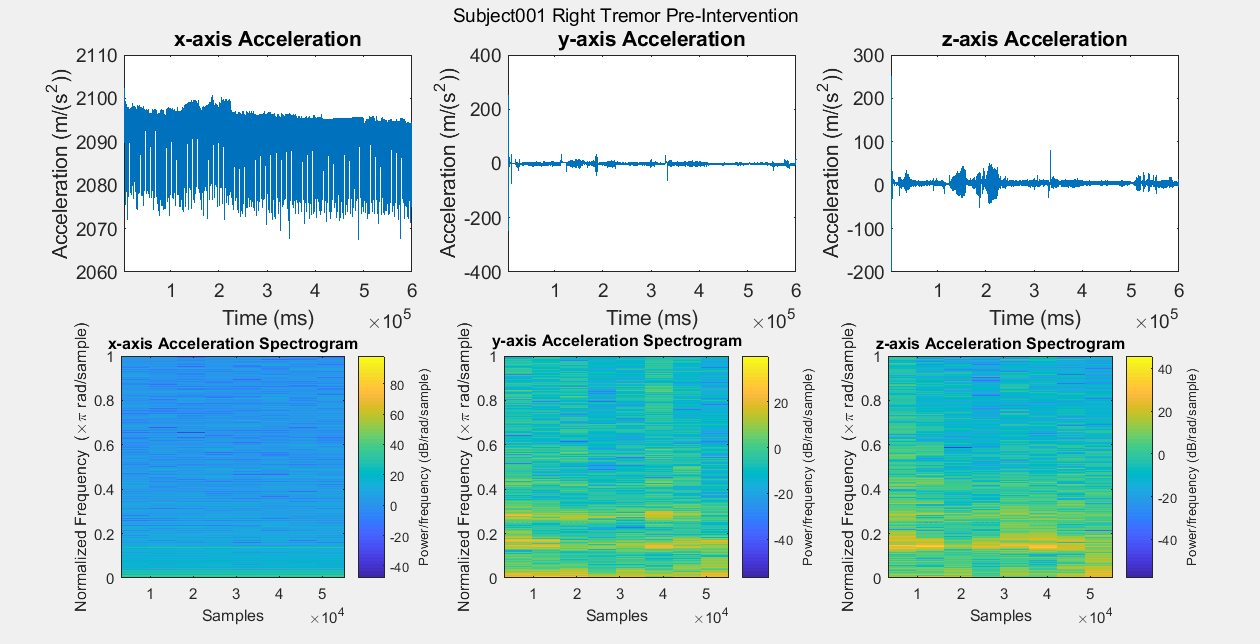
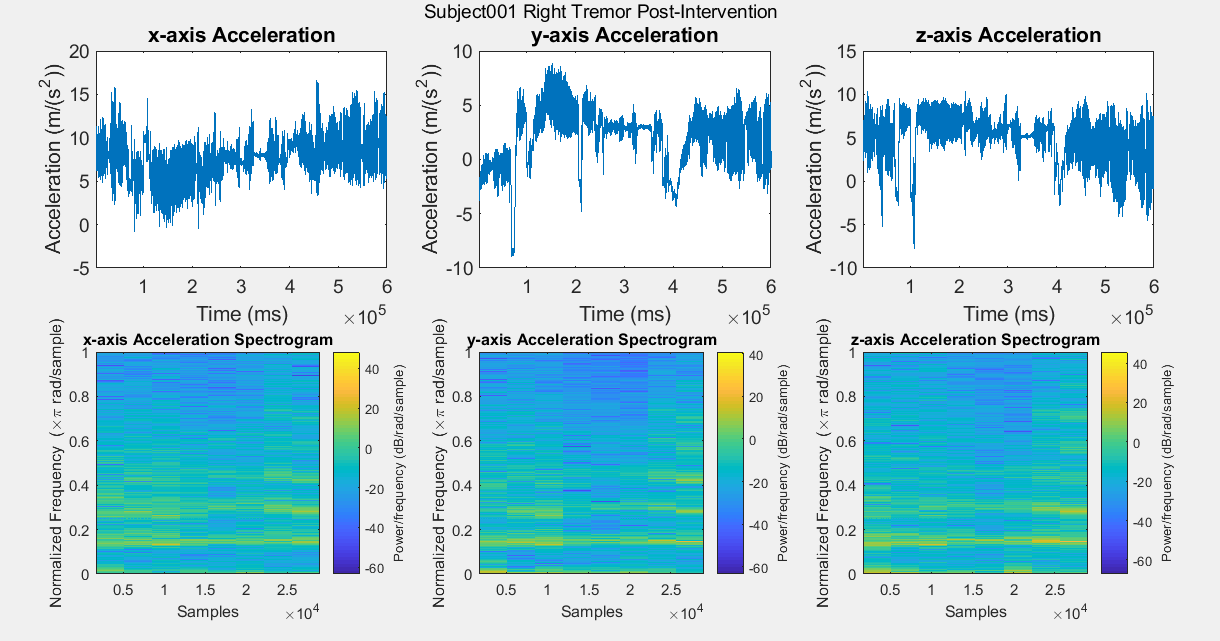
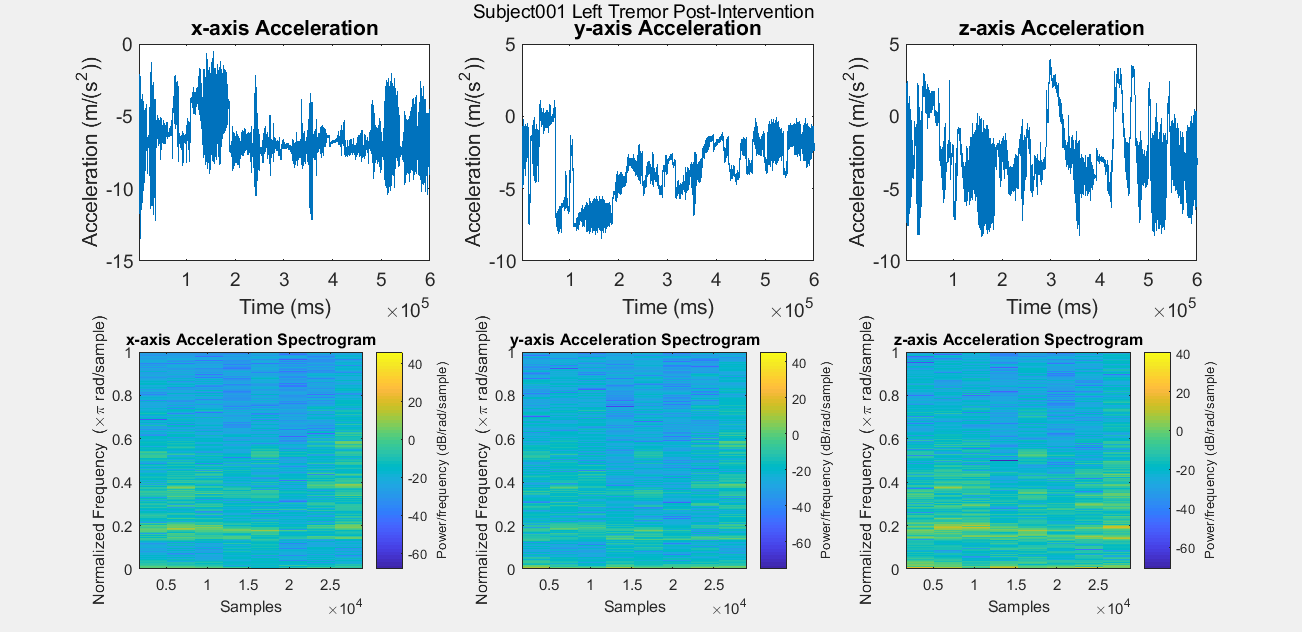
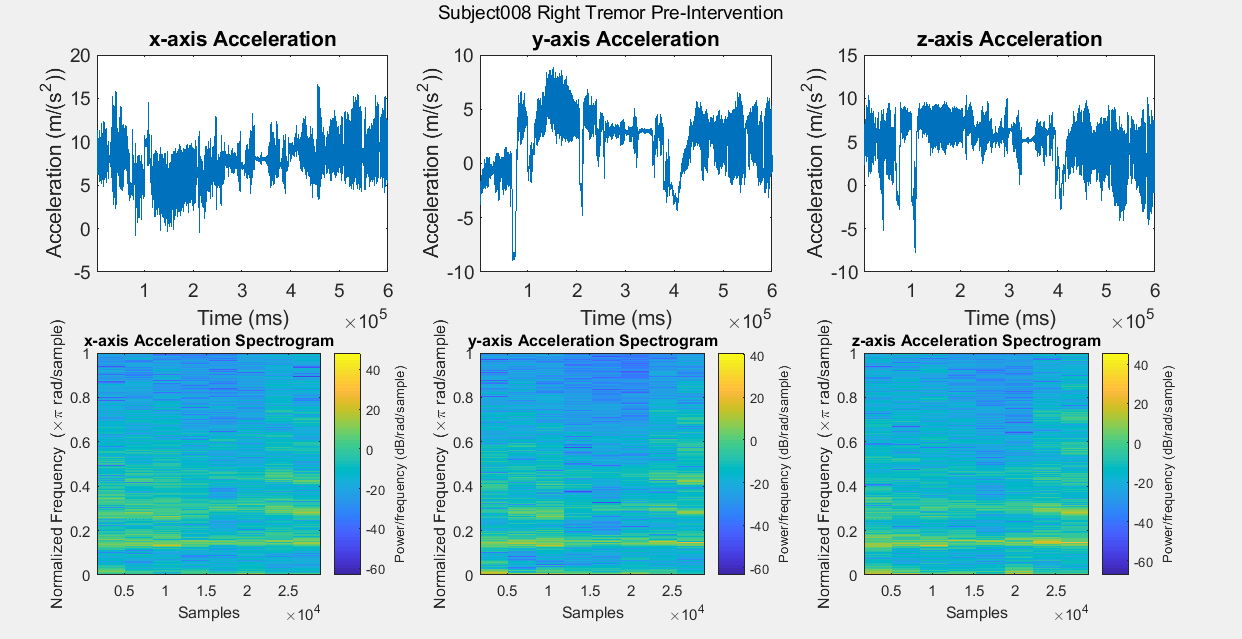
Results: Figure A is a Control non-PD subject without BEST intervention for baseline comparisons. The first row of plots are the subject’s xyz acceleration and the second row is the subject’s power density spectrograms. Figures B, C, D, and E show pre and post intervention RT data for control Subject001. Figures F and G show pre intervention RT data for experimental Subject008. With the current data, it is difficult to identify a center frequency for PD RT. However, it is possible to infer the hand that has the dominant tremor in each subject. In addition, subtle improvements can be observed in Subject001’s power spectrum. When comparing Figure C with Figure E, the subject’s spectrum loses some power at many different frequencies in the y and z components of acceleration.
Conclusions: Further investigation is required.
References: 1. Camara, C., Isasi, R., Warwick, K., Ruiz, V., Aziz, T., Stein, J., & Bakstein, E. (2014). Resting tremor classification and detection in Parkinson’s disease patients. Biomedical Signal Processing and Control, 16, 88-97. 2. Rigas, G., Gatsios, D., Fotiadis, D., Chondrogiorgi, M., Tsironis, C., Konitsiotis, S., Gentile, G., Marcante, A., & Antonini, A. (2016). Tremor UPDRS estimation in home environment. Engineering in Medicine and Biology Society, 3642-3645. 3. Alhamid, M., Alamri, A., & El Saddik, A. (2010). Measuring hand-arm steadiness for post-stroke and Parkinson’s disease patients using sierra framework. Medical measurements and applications proceedings (MeMeA). 4. Barth, J., Sunkel, M., Bergner, K., Schickhuber, G., Winkler, J., Klucken, J., & Eskofier, B. (2012). Combined analysis of sensor data from hand and gait motor function improves automatic recognition of Parkinson’s disease. Engineering in Medicine and Biology Society (EMBC), 5122-5125. 5. National Parkinson’s Disease Foundation. (2017). Treatment. Retrieved from http://www.parkinson.org/understanding-parkinsons/treatment. 6. Giuberti, M., Ferrari, G., Contin, L, Cimolin, V., Corrado, A., Albani, G., & Mauro, A. (2014). Linking UPDRS scores and kinematic variables in the leg agility task of parksonians. Wearable and Implantable Body Sensor Networks (BSN), 115-120. 7. Schon, K. (2017). Bio Electro Stimulation Therapy for Parkinson’s Disease. Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT03014050. 8. Schon, K. (2017). Bio Electro Stimulation Therapy for Parkinson’s Disease. Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT03014050. 9. Barth, J., Klucken, J., Kugler, P., Kammerer, T., Steidl, R., Winkler, J., Hornegger, J., & Eskofier, B. (2011). Biometric and mobile gait analysis for early diagnosis and therapy monitoring in Parkinson’s disease. Engineering in Medicine and Biology Society (EMBC), 868-871. 10. Klucken, J., Barth, J., Kugler, P., Schlachetski, J., Henze, T., Marxreiter, F., Kohl, Z., Steidl, R., Hornegger, J., Eskofier, B., & Winkler, J. (2013). Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. Public Library of Science (PLoS One), 8, 1-9. 11. El-Gohary, M., McNames, J., Chung, K., Aboy, M., Salarian, A., & Horak, F. (2010) Continuous at-home monitoring of tremor in patients with Parkinson’s disease. Analysis of Biomedical Signals and Images, 20, 420-424. 12. Dai, H., Zhang, P., & Lueth, T. (2015). Quantitative assessment of parksonian tremor based on inertial measurement unit. Sensors, 15, 25055-25071. 13. Tzallas, A., Tsipouras, M., Rigas, G., Tsalikakas, D., Karvounis, E., Chondrogiorgi, M., Psomadellis, F., Cancela, J., Pastorino, M., Waldmeyer, M., Konitsiotis, S., & Fotiadis, D. (2014). PERFORM: A system for monitoring, assessment and management of patients of patients with Parkinson’s Disease. Sensors, 14, 21329-21357. 14. Darnall, N., Donovan, C., Tseng, H., Barthelmess, P., Cohen, & Lin, D. (2012). Application of machine learning and numerical analysis to classify tremor in patients affected with essential tremor or Parkinson’s disease. Gerontechnology, 10, 208-219. 15. Eskofier, B., Lee, S., Daneault, J., Golabchi, F., Ferreira-Carvalho, G., Sapienza, S., Constante, G., Klucken, J., Kautz, T., & Bonato, P. (2016). Recent machine learning advancements in sensor-based mobility analysis: deep learning for Parkinson’s disease assessment. Engineering in Medicine and Biology Society (EMBC), 655-658. 16. Barth, J., Klucken, J., Kugler, P., Kammerer, T., Steidl, R., Winkler, J., Hornegger, J., & Eskofier, B. (2011). Biometric and mobile gait analysis for early diagnosis and therapy monitoring in Parkinson’s disease. Engineering in Medicine and Biology Society (EMBC), 868-871. 17. Klucken, J., Barth, J., Kugler, P., Schlachetski, J., Henze, T., Marxreiter, F., Kohl, Z., Steidl, R., Hornegger, J., Eskofier, B., & Winkler, J. (2013). Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. Public Library of Science (PLoS One), 8, 1-9. 18. Pansera, M., Estrada, J., Pastor, L., Cancela, J., Greenlaw, R., & Arrendondo, M. (2009). Multi-parametric system for the continuous assessment and monitoring of motor status in Parkinson’s disease: an entropy-based gait comparison. Engineering in Medicine and Biology Society (EMBC), 1242-1245. 19. Darnall, N., Donovan, C., Tseng, H., Barthelmess, P., Cohen, & Lin, D. (2012). Application of machine learning and numerical analysis to classify tremor in patients affected with essential tremor or Parkinson’s disease. Gerontechnology, 10, 208-219. 20. Rigas, G., Tzallas, A., Tsalikakis, D., Konitsiotis, S., & Fotiadis, D. (2009) Real-time quantification of resting tremor in the Parkinson’s disease. Engineering in Medicine and Biology Society (EMBC), 1306-1309. 21. Klucken, J., Barth, J., Kugler, P., Schlachetski, J., Henze, T., Marxreiter, F., Kohl, Z., Steidl, R., Hornegger, J., Eskofier, B., & Winkler, J. (2013). Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. Public Library of Science (PLoS One), 8, 1-9. 22. Rigas, G., Gatsios, D., Fotiadis, D., Chondrogiorgi, M., Tsironis, C., Konitsiotis, S., Gentile, G., Marcante, A., & Antonini, A. (2016). Tremor UPDRS estimation in home environment. Engineering in Medicine and Biology Society, 3642-3645. 23. Rigas, G., Tzallas, A., Tsalikakis, D., Konitsiotis, S., & Fotiadis, D. (2009) Real-time quantification of resting tremor in the Parkinson’s disease. Engineering in Medicine and Biology Society (EMBC), 1306-1309. 24. Rigas, G., Gatsios, D., Fotiadis, D., Chondrogiorgi, M., Tsironis, C., Konitsiotis, S., Gentile, G., Marcante, A., & Antonini, A. (2016). Tremor UPDRS estimation in home environment. Engineering in Medicine and Biology Society, 3642-3645. 25. Yang, J., Chang, R., Chen, F., Chern, C., & Chiu, J. (2016). Detection of hand tremor in patients with Parkinson’s disease using a non-invasive laser line triangulation measurement method. Measurement, 79, 20-28. 26. Camara, C., Isasi, R., Warwick, K., Ruiz, V., Aziz, T., Stein, J., & Bakstein, E. (2014). Resting tremor classification and detection in Parkinson’s disease patients. Biomedical Signal Processing and Control, 16, 88-97. 27. Lambrecht, S., Gallego, J., Rocon, E., & Pons, J. (2014). Automatic real-time monitoring and assessment of tremor parameters in the upper limb from orientation data. Frontiers in Neuroscience, 8, 1-9. 28. Jitkritsadakul, O., Thanawattano, C., Anan, C., & Bhidayasiri, R. (2015). Exploring the effect of electrical muscle stimulation as a novel treatment of intractable tremor in Parkinson’s disease. Journal of the Neurological Sciences, 358, 146-152. 29. Camara, C., Isasi, R., Warwick, K., Ruiz, V., Aziz, T., Stein, J., & Bakstein, E. (2014). Resting tremor classification and detection in Parkinson’s disease patients. Biomedical Signal Processing and Control, 16, 88-97. 30. Patrick, S.K., Dennington, A. A., Gauthier, M. J. A., Gillard, G. M., & Prochazka, A. (2001). Quantification of the UPDRS rigidity scale. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 9, 31-41. 31. Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stern, M. B., Dodel, R., Dubois, B., Holloway, R., Jankovic, J., Kulisevsky, J., Lang, A. E., Lees, A., Leurgans, S., LeWitt, P. A., Nyenhuis, D., Olanow, C. W., Rascol, O., Schrag, A., Teresi, J. A., van Hilten, J. J. & LaPelle, N. (2008), Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord., 23: 2129–2170. doi:10.1002/mds.22340. 32. Fahn, S.; Elton, R.L. Unified Parkinson’s Disease Rating Scale. Recent Developments in Parkinson’s Disease, Available online: http://img.medscape.com/fullsize/701/816/58977_UPDRS.pdf. 33. Schon, K. (2017). Bio Electro Stimulation Therapy for Parkinson’s Disease. Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT03014050. 34. Dai, H., Zhang, P., & Lueth, T. (2015). Quantitative assessment of parksonian tremor based on inertial measurement unit. Sensors, 15, 25055-25071. 35. Klucken, J., Barth, J., Kugler, P., Schlachetski, J., Henze, T., Marxreiter, F., Kohl, Z., Steidl, R., Hornegger, J., Eskofier, B., & Winkler, J. (2013). Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. Public Library of Science (PLoS One), 8, 1-9. 36. Jitkritsadakul, O., Thanawattano, C., Anan, C., & Bhidayasiri, R. (2015). Exploring the effect of electrical muscle stimulation as a novel treatment of intractable tremor in Parkinson’s disease. Journal of the Neurological Sciences, 358, 146-152.
To cite this abstract in AMA style:
T. Tsao, C. Fontana, A. Waite. Bio-Electro Stimulation Therapy for the Treatment of Motor and Non-Motor Symptoms in Parkinson’s Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/bio-electro-stimulation-therapy-for-the-treatment-of-motor-and-non-motor-symptoms-in-parkinsons-disease/. Accessed April 1, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/bio-electro-stimulation-therapy-for-the-treatment-of-motor-and-non-motor-symptoms-in-parkinsons-disease/