Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To evaluate the efficacy of CVXL-0107 on motor symptoms and on levodopa-induced dyskinesia in advanced Parkinson’s disease (PD) patients.
Background: CVXL-0107 is a glutamate release inhibitor which has shown evidence of antiparkinsonian and antidyskinetic activities in a non-human primate model, and in a previous proof of concept study in PD.(1)
Methods: A multicenter, cross-over, double-blind, randomized, placebo-controlled trial was conducted by the French NS-PARK/FCRIN network (NCT02641054). Advanced PD patients with motor fluctuations and dyskinesia were submitted to 14-day treatment periods with CVXL-0107 40 mg q.i.d or placebo followed by an acute challenge of levodopa + CVXL-0107 or levodopa + placebo. The 2 co-primary endpoints were the areas under the curve (AUC) of (1) the MDS-UPDRS Part III (motor status) and (2) AIMS scores (dyskinesia) evaluated at baseline, 20, 40, 60, and then every 30 minutes until OFF state return. Total MDS-UPDRS, UDysRS, Hauser patient diary, levodopa and CVXL-0107 pharmacokinetics, and safety were also assessed.
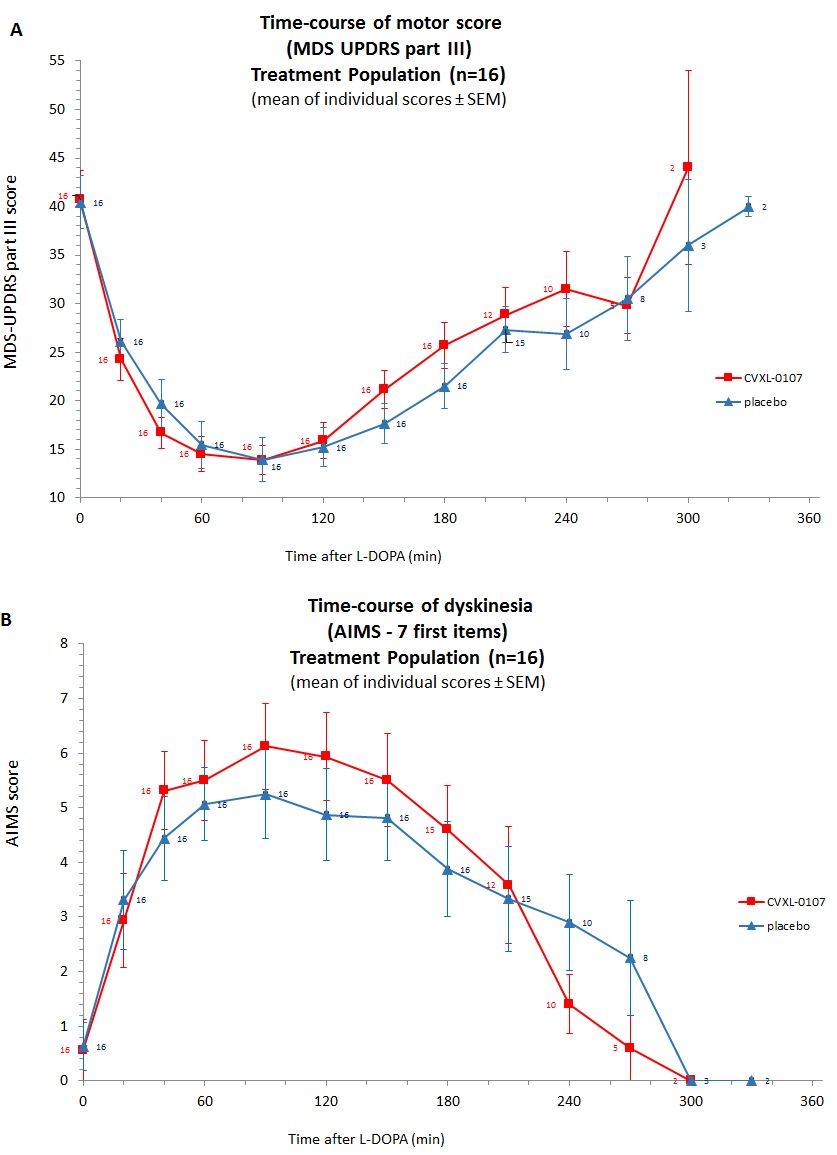
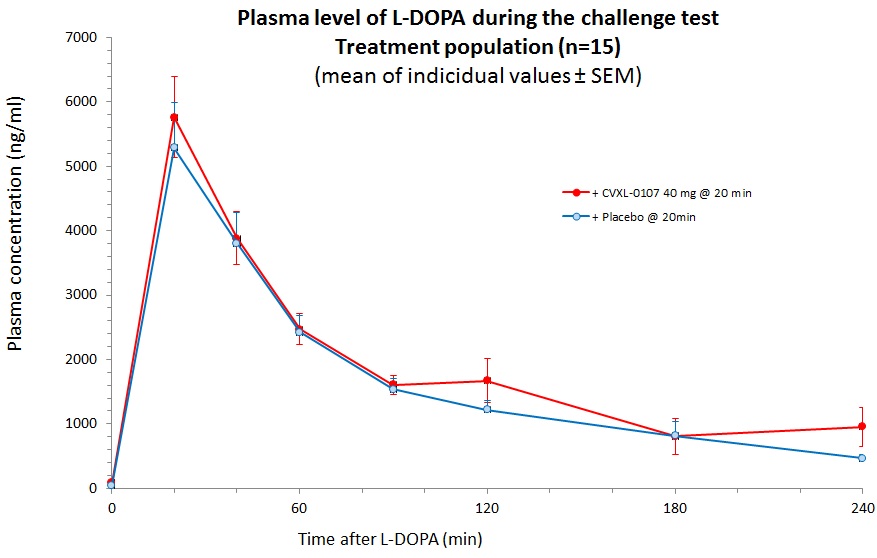
Results: The primary analysis population was composed of 16 patients equally (8:8) allocated in the 2 treatment sequence groups. AUC0-t of either MDS-UPDRS Part III scores or AIMS scores showed no significant difference between placebo and CVXL-0107 (Fig 1, parts A & B, p=0.85 and p=0.57 for motor and dyskinesia AUCs respectively). [figure1] The levodopa plasma concentrations reached a peak at a similar median tmax (20 min) and declined with a similar mean terminal half-life (67.2-69,6 min) (Fig 2). CVXL-0107 median tmax (141 min) occurred much later than that of levodopa. [figure2] No significant difference in favor to CVXL-0107 was observed regarding secondary endpoints. A trend in favor of CVXL-0107 was however observed on axial motor scores. CVXL-0107 was well tolerated, there was no SAE, no study withdrawal, and no treatment interruption.
Conclusions: We did not find efficacy of CVXL-0107 on neither motor nor dyskinesia primary endpoints in this trial. These negative findings may be explained by an inappropriate dose and/or a mismatch between L-DOPA and CVXL-0107 PK profiles during the challenge test.
References: (1) A proof-of-concept, randomized, placebo-controlled, multiple cross-overs (n-of-1) study of naftazone in Parkinson’s disease. Rascol O, Ferreira J, Nègre-Pages L, Perez-Lloret S, Lacomblez L, Galitzky M, Lemarié JC, Corvol JC, Brotchie JM, Bossi L. Fundam Clin Pharmacol. 2012 Aug;26(4):557-64.
To cite this abstract in AMA style:
JC. Corvol, F. Durif, W. Meissner, JP. Azulay, R. Haddad, D. Bricout, X. Wang-Zhang, L. Benel, O. Rascol, Fr. NS-Park/F-CRIN Network. A Double-Blind, Randomized, Placebo-Controlled, Cross-Over Phase IIa Trial to Evaluate the Efficacy of CVXL-0107 on Parkinson-Related Symptoms and Levodopa-Induced Dyskinesia in Advanced Parkinson’s Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/a-double-blind-randomized-placebo-controlled-cross-over-phase-iia-trial-to-evaluate-the-efficacy-of-cvxl-0107-on-parkinson-related-symptoms-and-levodopa-induced-dyskinesia-in-advanced-parkinson/. Accessed December 27, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-double-blind-randomized-placebo-controlled-cross-over-phase-iia-trial-to-evaluate-the-efficacy-of-cvxl-0107-on-parkinson-related-symptoms-and-levodopa-induced-dyskinesia-in-advanced-parkinson/