Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To investigate whether women and men with Parkinson’s disease (PD) may differ in their biochemical and clinical responses to long-term treatment with urate-elevating inosine.
Background: Higher levels of serum or CSF urate predict a reduced risk of PD or a favorable rate of its progression (especially in men), and can confer protection against dopaminergic neuron degeneration in preclinical PD models. These findings have prompted clinical development of inosine as a urate-elevating strategy with the potential to slow PD progression. Because women on average have lower urate levels than men, a treatment that targets the same range of higher urate for both sexes should lead to a greater mean increase in women.
Methods: The SURE-PD (phase 2) trial enrolled 75 people with early PD and baseline serum urate below 6 mg/dL and randomized them to three double-blinded treatment arms: placebo or oral inosine titrated to produce mild (6.1-7.0 mg/dL) or moderate (7.1-8.0 mg/dL) serum urate elevation for up to two years. Here we report secondary analyses stratified by sex
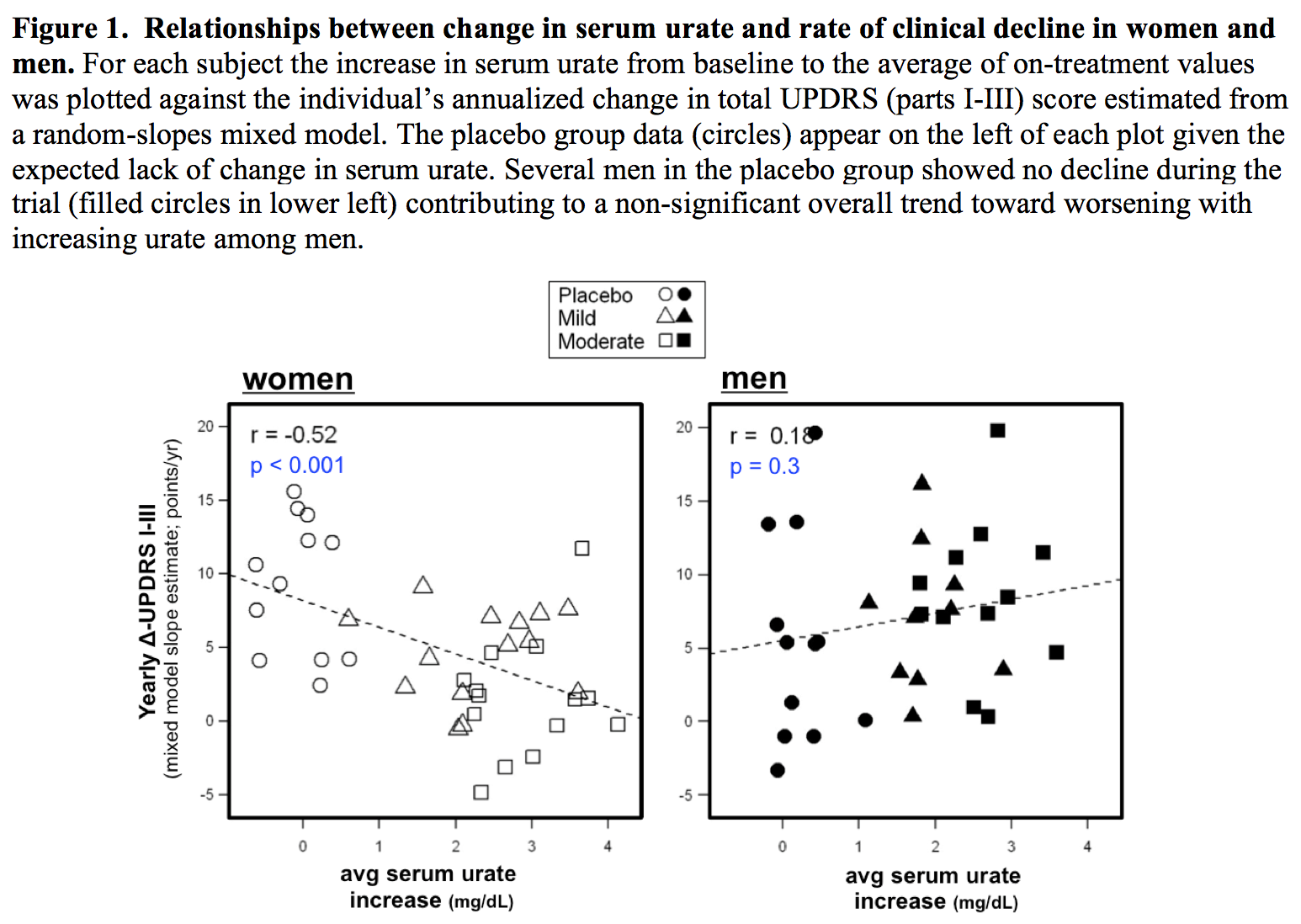
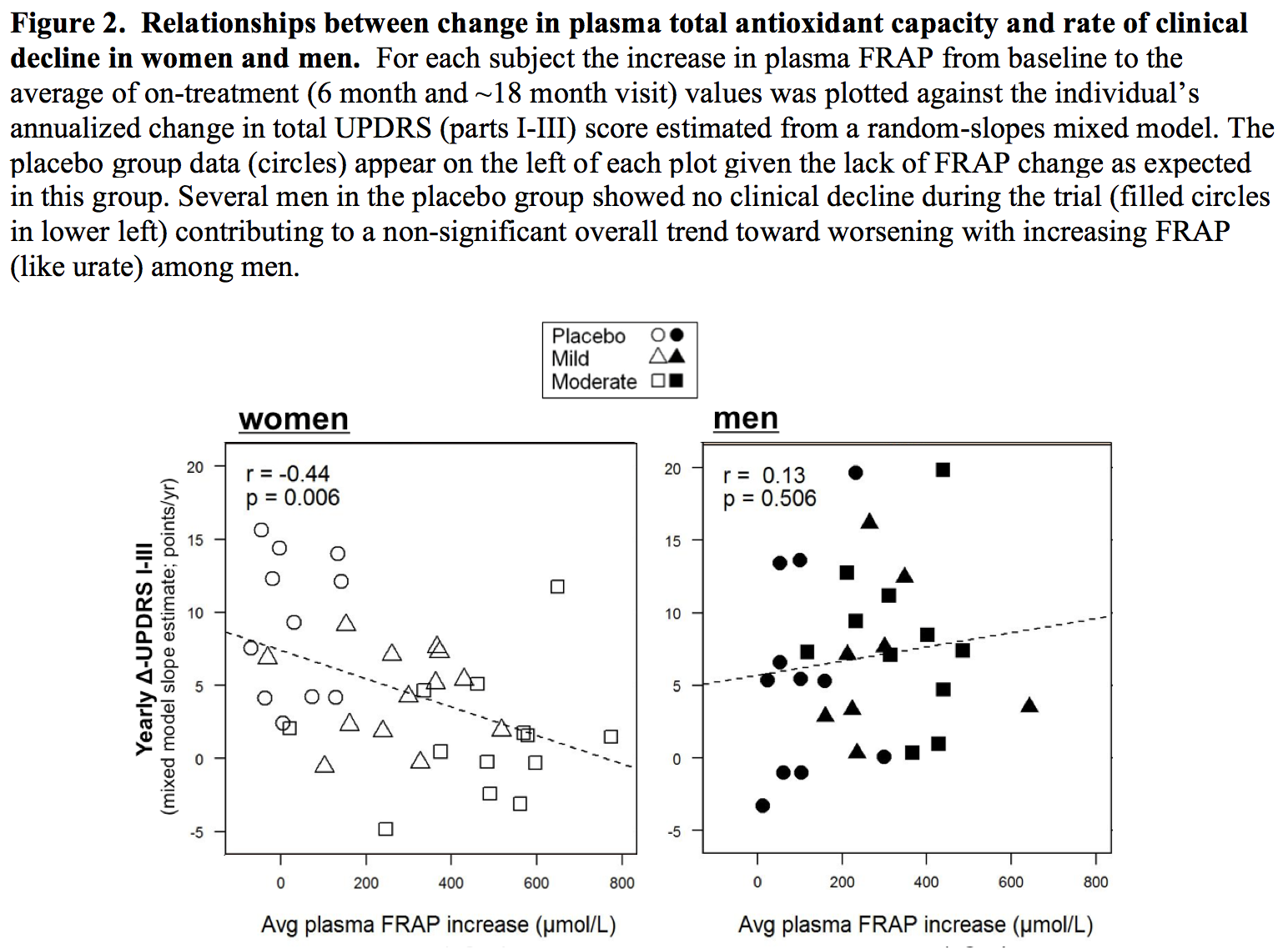
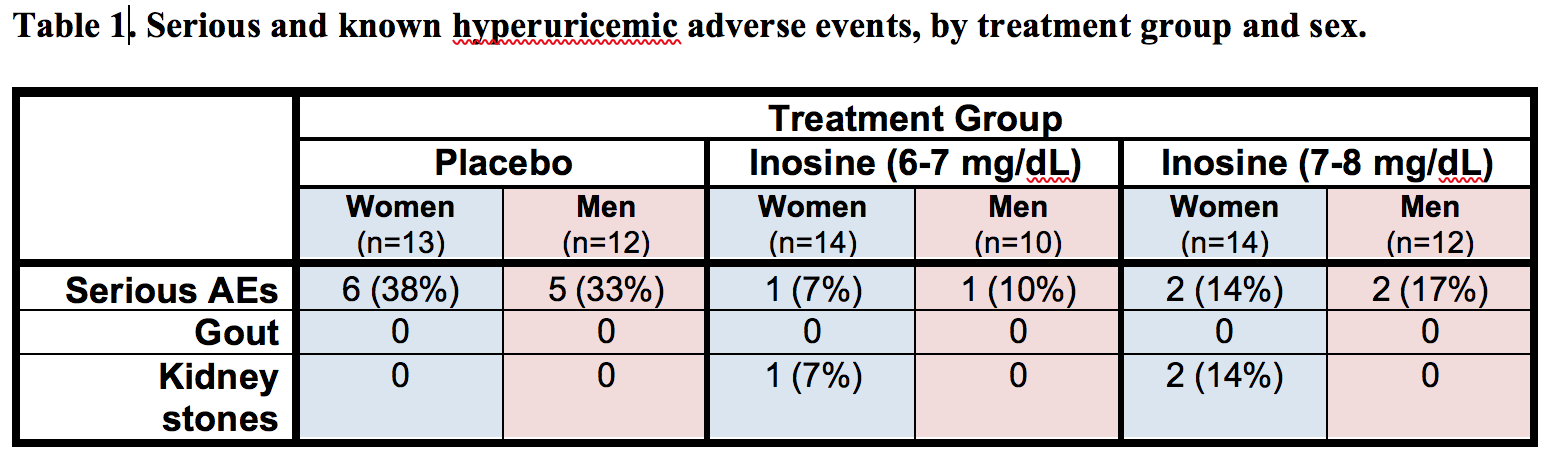
Results: The absolute increase in serum urate induced by inosine was 20% greater in women, consistent with their expected lower urate levels at baseline. Similarly, only among women was CSF urate significantly greater on mild or moderate inosine (+88% [p<0.001] and +98% [p<0.001], respectively) compared to placebo (in contrast to men: +10% [p=0.6] and +14% [p=0.4], respectively). The previously reported trend toward slower clinical progression with increasing inosine is attributable to slower decline in women, based on slower worsening of Unified PD Rating Scale [UPDRS] score with higher inosine dosing (7.0 points/year slower rate of change on moderate inosine versus placebo; p = 0.01), with greater urate elevation (r = -0.52; p = 0.001; [figure1]), or with greater increase in plasma antioxidant capacity (r = -0.44; p = 0.006; [figure2]), whereas no significant difference was observed in men. Although kidney stones developed only among those treated with inosine and only among women, overall incidence of serious adverse events was no higher among women than men [table1].
Conclusions: Inosine produced greater increases in serum and CSF urate in women compared to men in the SURE-PD trial, consistent with the study’s design. Preliminary evidence suggests that urate-elevating inosine treatment may slow clinical decline in early PD primarily or only among women.
To cite this abstract in AMA style:
M. Schwarzschild, E. Macklin, R. Bakshi, S. Bhattacharyya,, R. Logan, A. Ascherio; on behalf of the Parkinson Study Group SURE-PD Investigators. Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/sex-differences-by-design-and-outcome-in-the-safety-of-urate-elevation-in-pd-sure-pd-trial/. Accessed December 22, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/sex-differences-by-design-and-outcome-in-the-safety-of-urate-elevation-in-pd-sure-pd-trial/