Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: Evaluate efficacy/safety of levodopa-carbidopa intestinal gel (LCIG) daytime monotherapy (with/without nighttime oral carbidopa/levodopa) vs polytherapy (LCIG with >1 adjunctive therapy) in advanced Parkinson’s disease (PD) patients.
Background: Treating motor complications often requires adjunctive PD therapies. Continuous LCIG administration reduces motor complications associated with oral levodopa, while potentially reducing pill burden.
Methods: LCIG was administered continuously 16 hours/day via percutaneous endoscopic gastrojejunostomy (PEG-J) in 3 phase 3 studies. The first was a 52-week open-label extension of a 12-week double-blind study in which patients received LCIG or oral carbidopa/levodopa. In the extension, both treatment groups received LCIG. The second was a 54-week, open-label study of LCIG. Patients from these 2 studies could continue LCIG in an extended-access study, until commercially available. Efficacy and safety in patients on daytime LCIG monotherapy vs polytherapy were assessed.
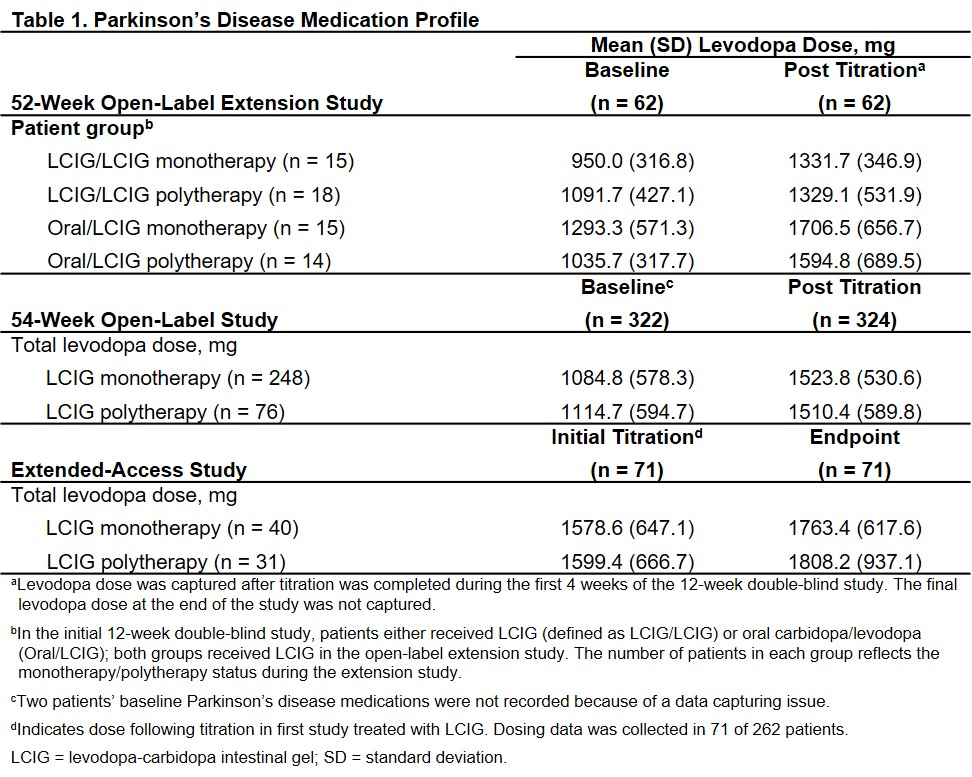
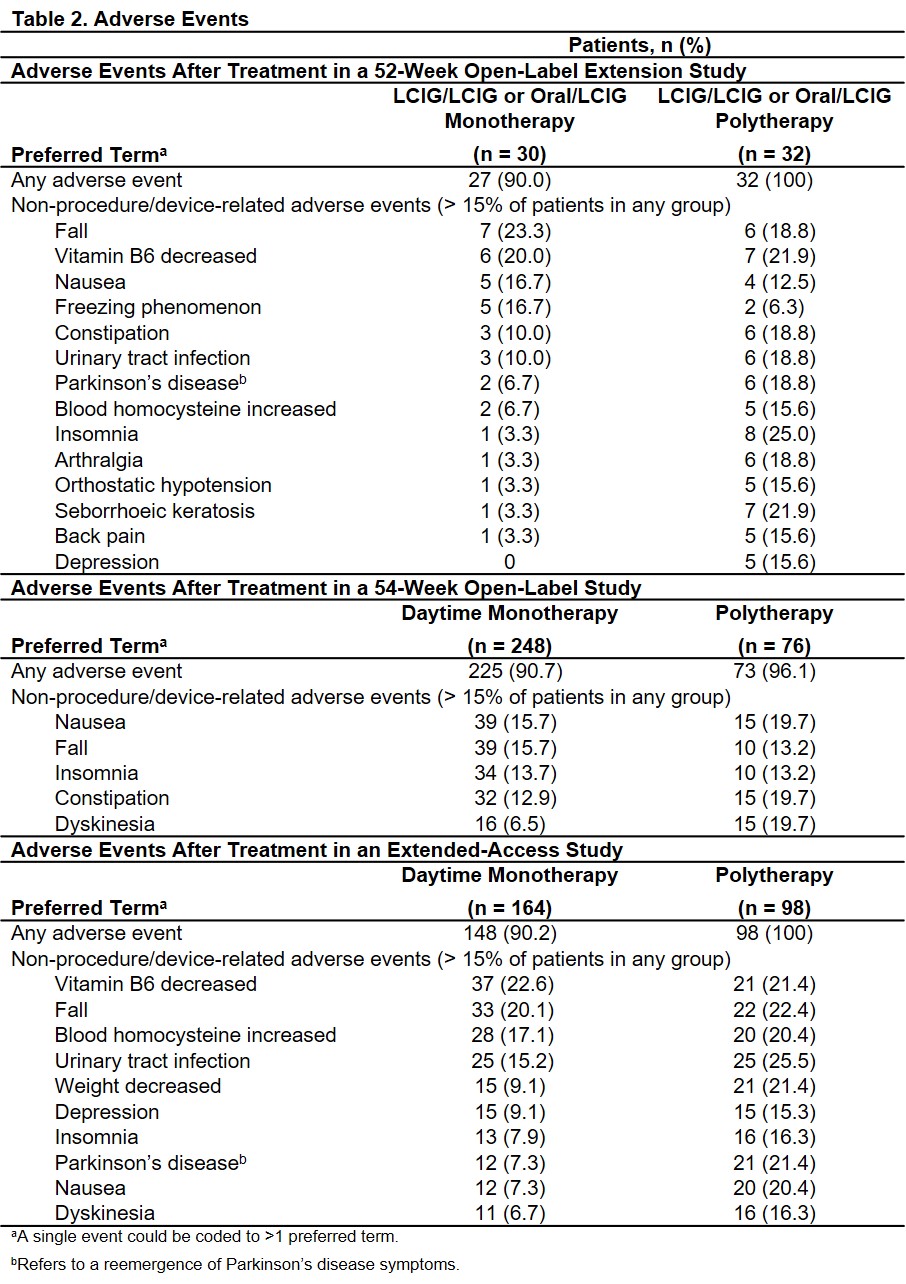
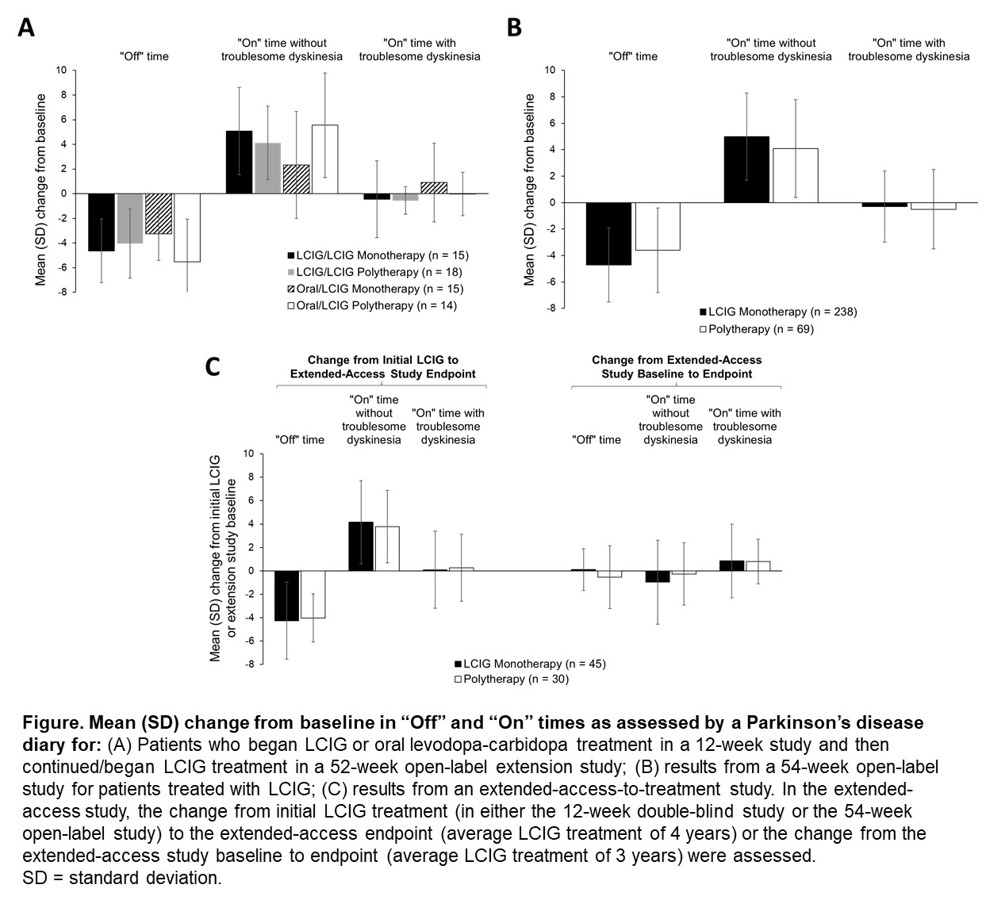
Results: Across 3 studies, 386 patients received LCIG via PEG-J for a mean duration of 3.0 years (range: 1 day to 6.9 years). In the first study, 30 patients were on LCIG daytime monotherapy and 32 patients were on LCIG polytherapy. In the second study, of 324 patients who had PEG-J placement, 248 (76.5%) were on LCIG daytime monotherapy (of these, 90 received no overnight oral carbidopa/levodopa). In the extended-access study, 164 of 262 were on LCIG daytime monotherapy; (75 with efficacy data of which 45 [60%] were on LCIG daytime monotherapy). Total daily levodopa dose increased with LCIG use in all groups.[Table 1] In these studies, patients on daytime LCIG monotherapy vs polytherapy experienced similar reductions in “Off” time and improvements in “On” time.[Figure] Adverse events were similar for patients on LCIG daytime monotherapy vs polytherapy in each study.[Table 2]
Conclusions: As PD progresses, the number of anti-Parkinsonian medications and frequency of dosing tends to increase, which correlates with decreased patient compliance and suboptimal control of PD symptoms.[Ref1&2] Daytime LCIG monotherapy and polytherapy demonstrated similar efficacy and safety profiles in 3 phase 3 studies in advanced PD patients, suggesting that LCIG monotherapy can provide a more simplified treatment option with similar efficacy for appropriate patients.
References: Ref1: Fleisher and Stern, Curr Neurol Neurosci Rep. 2013;13:382. Ref2: Davis et al, Mov Disord. 2010;25:474.
To cite this abstract in AMA style:
J. Boyd, C. Zadikoff, M. Siddiqui, J. Benesh, J. Zamudio, W. Robieson, P. Kukreja. Safety and Efficacy of Levodopa-Carbidopa Monotherapy in Patients with Advanced Parkinson’s Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-levodopa-carbidopa-monotherapy-in-patients-with-advanced-parkinsons-disease/. Accessed December 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-levodopa-carbidopa-monotherapy-in-patients-with-advanced-parkinsons-disease/