Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To review the design and unique features of an ongoing phase 3 trial of a gastric-retentive formulation of carbidopa/levodopa (CD/LD), the Accordion Pill™ (AP), versus immediate-release (IR)-CD/LD (Sinemet®) in patients with PD experiencing motor fluctuations.
Background: PD patients eventually require LD treatment. Many experience dose-by-dose motor fluctuations and have substantial daily OFF time. AP-CD/LD is a novel gastric-retention pill with multilayer films containing IR CD and both IR and controlled-release LD to improve drug delivery. Pharmacokinetic studies with AP-CD/LD suggest improvements over IR-CD/LD.
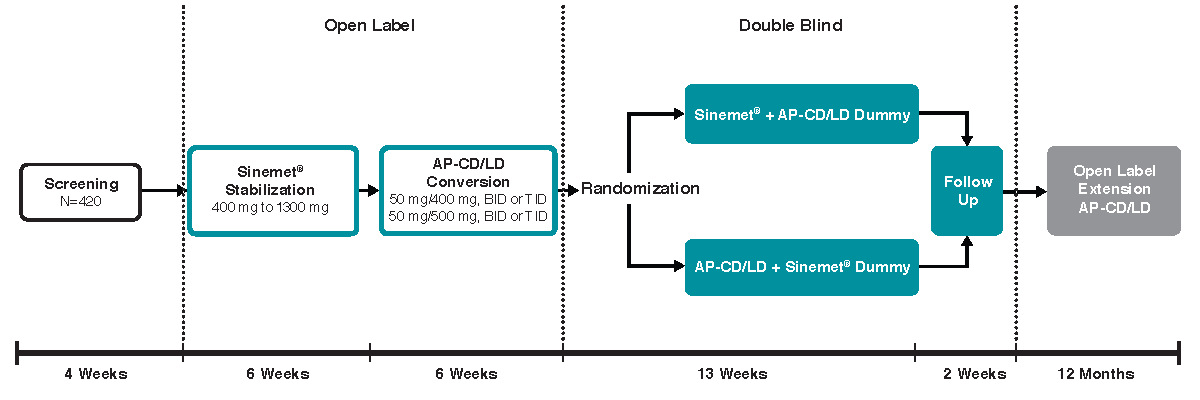
Methods: Study IN-11-004 (NCT02605434) is a randomized, double blind, active controlled, parallel group study enrolling PD patients with motor fluctuations. Eligible patients require daily LD intake of 400 mg to 1300 mg or equivalent and experience ≥2.5 hours OFF time daily. The study includes two unique, 6-week, open label, dose titration phases: the first 6 weeks is dose stabilization of the active comparator, IR-CD/LD, and the second 6 weeks is conversion to AP-CD/LD [figure1]. Following open label dose titration, patients are randomized to double blind, 13-week maintenance treatment with either IR-CD/LD or AP-CD/LD, followed by a 2-week follow-up period. The primary endpoint is change in total OFF time (as a percent of waking hours). Secondary endpoints include: daily OFF time (h); ON time (h with and without troublesome dyskinesia); number of daily LD doses; Clinician’s Global Impression–Global Improvement (CGI-I); Parkinson’s Disease Questionnaire–39 items (PDQ-39); Parkinson’s Disease Sleep Scale (PDSS); and Unified Parkinson’s Disease Rating Scale (UPDRS). Safety is assessed via adverse event monitoring and standard clinical and laboratory procedures.
Results: In mid-2016, approximately 70 study centers in the United States, Europe, and Israel began recruitment. Full study enrollment is estimated during the second half of 2018. Following successful study completion, each patient can enroll in a 12-month open label safety extension with AP-CD/LD.
Conclusions: This phase 3 randomized, double blind, active controlled study of AP-CD/LD vs CD/LD has unique design features, including an open label dose stabilization and conversion phase and treatment with an active comparator (IR-CD/LD).
To cite this abstract in AMA style:
P. LeWitt, R. Gendreau, J. Meckler, N. Navon. Design of a Phase 3 Efficacy and Safety Trial of Accordion Pill™ Carbidopa/Levodopa for Parkinson’s Disease (PD) Patients Experiencing Motor Fluctuations [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/design-of-a-phase-3-efficacy-and-safety-trial-of-accordion-pill-carbidopa-levodopa-for-parkinsons-disease-pd-patients-experiencing-motor-fluctuations/. Accessed December 30, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/design-of-a-phase-3-efficacy-and-safety-trial-of-accordion-pill-carbidopa-levodopa-for-parkinsons-disease-pd-patients-experiencing-motor-fluctuations/