Session Information
Date: Saturday, October 6, 2018
Session Title: Clinical Trials and Therapy in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To utilize clinical, imaging and CSF data from the Parkinson’s Progression Markers Initiative (PPMI) to estimate the sample size for disease modification studies in Parkinson’s disease (PD) participants.
Background: The PPMI study provides longitudinal clinical and biomarker data that may be utilized to design PD therapeutic trials to assess putative disease modifying therapies. A major roadblock for these PD studies is to accurately establish the sample size required to establish drug effect and dosage in a reasonably sized Phase 2 program.
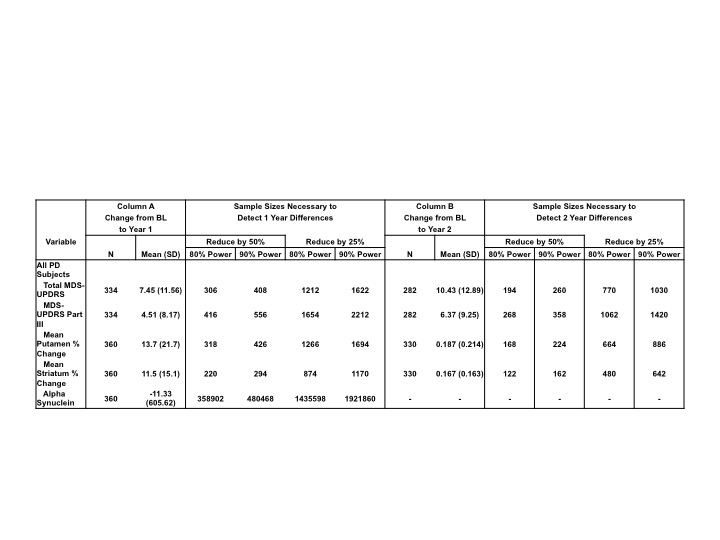
Methods: PPMI PD and healthy control participants are evaluated annually with a wide range of assessments including the MDS-UPDRS, DAT/VMAT imaging and CSF measures. PPMI subjects have been followed for more than five years. We utilized PPMI longitudinal data to establish sample size estimates for these measures for studies of up to two years duration assessing PD subjects. Given the large confound of MDS-UPDRS in subjects treated with dopaminergic therapies, we examined the sample size in treated and untreated PD after 12 month of PPMI participation. In addition, since an exponential fit of DAT imaging data provided a better fit than a linear model, we have used the exponential model to explore sample size estimates.
Results: Table 1 shows the one and two year sample size for all PD subjects evaluated for one and two years. Of the variables assessed mean putamen and striatum DAT scores allow the smallest sample size. Additional data demonstrate that DAT imaging sample size estimates may be substantially improved by utilizing an exponential fit of three scans. VMAT2 imaging may provide an alternative imaging biomarker with improved sample size estimates but additional data are required. MDS-UPDRS I-III untreated reduces sample size compared to the UPDRS for the entire group. Extending the follow up to 2 years would allow reducing the sample size by close to 50%. CSF measures would require a substantially larger sample size.
Conclusions: Sample size data can be utilized in planning future clinical trials of potential disease modifying interventions in PD. Powering the study based on DAT imaging utilizing an exponential fit can substantially reduce the sample size in the treated PD population. Extending the duration of the study to 2 years will also reduce the sample size but has to be balanced with the challenges of retention and overall cost.
To cite this abstract in AMA style:
K. Marek, J. Seibyl, C. Caspell-Garcia, C. Coffey, B. Mollenhauer, K. Kieburtz, C. Tanner, L. Chahine, A. Siderowf, T. Simuni. PPMI driven sample size estimation for clinical trials in Parkinson’s disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/ppmi-driven-sample-size-estimation-for-clinical-trials-in-parkinsons-disease/. Accessed April 1, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/ppmi-driven-sample-size-estimation-for-clinical-trials-in-parkinsons-disease/