Session Information
Date: Saturday, October 6, 2018
Session Title: Clinical Trials and Therapy in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: The objective of this study is to access the efficacy of CX-8998 using the MDS-UPDRS Tremor Score (MDS-UPDRS-TS) and a digital biomarker platform, while evaluating safety, tolerability and plasma concentrations of CX-8998.
Background: Parkinson’s disease (PD) is a neurodegenerative disease that affects over 6 million people worldwide. PD is characterized by the classic motor symptom triad of resting tremor, rigidity and bradykinesia. Rest tremor is one, but not the only, tremor variant in PD. Collectively, all tremor types in PD are termed as PD Tremor (PDT). PDT can be very disabling, especially if a postural and kinetic component exist. There are no approved treatments specifically for PDT. Most patients only respond modestly to first line anti-parkinsonian medications, and even fewer respond to second-line medications that are typically used in patients with essential tremor. Tremor resistant to oral medication remains a criterion to recommend patients for DBS surgery.
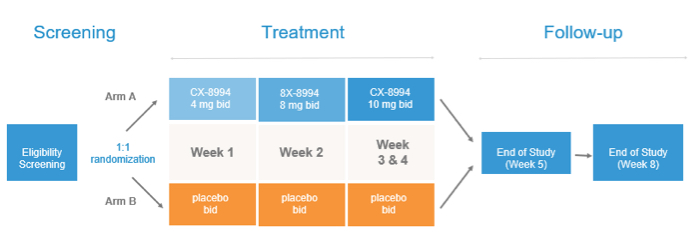
Methods: A randomized, double-blind, placebo-controlled, parallel-group study (NCT03436953) of 60 patients with PDT is underway to evaluate titrating doses of a selective Cav3 modulator, CX-8998. Subjects receive single oral doses of either CX-8998 up to 10mg BID) or placebo. The primary endpoint is change from baseline to Day 28 on the MDS-UPDRS Tremor Score assessed by a central rater and is powered to detect a 2.4-point difference between placebo and CX-8998.
Results: Dose selection was based on several preclinical models, a Ph1 pharmaco-EEG dataset that revealed statistically significant reductions of alpha power of ~25% or greater occur in a concentration-dependent manner at plasma CX-8998 concentrations greater than 200-300nM. The maximal reduction in alpha band power was observed at plasma CX-8998 concentrations of approximately 700-800 nM and greater. Safety/pharmacodynamic modeling also confirms our dose selection for T-CALM. Disclosure: Study Sponsored by Cavion Inc. Abstract presented at American Academy of Neurology Meeting, Los Angeles, CA, USA, April 2018.
Conclusions: This study is expected to test the hypothesis whether selective and potent modulation of Cav3 channels with CX-8998, can improve symptom severity in PDT patients.
To cite this abstract in AMA style:
S. Papapetropoulos, M. Lee, S. Boyer, M. Versavel. A Phase 2 Efficacy Study of CX-8998, a Potent and Selective T-Type Calcium (Cav3) Modulator in Parkinson’s disease Tremor (PDT) Patients: Design and Dose-Selection Rationale [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/a-phase-2-efficacy-study-of-cx-8998-a-potent-and-selective-t-type-calcium-cav3-modulator-in-parkinsons-disease-tremor-pdt-patients-design-and-dose-selection-rationale/. Accessed December 14, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-phase-2-efficacy-study-of-cx-8998-a-potent-and-selective-t-type-calcium-cav3-modulator-in-parkinsons-disease-tremor-pdt-patients-design-and-dose-selection-rationale/