Session Information
Date: Saturday, October 6, 2018
Session Title: Clinical Trials and Therapy in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: In clinical trials for new PD therapies, it is common for patients to start to take approved symptomatic PD medications during the study. The effect of these medications on disease progression need to be understood in order to better design PD clinical trials.
Background: PPMI is an ongoing observational study that enrolled 423 treatment-naïve PD subjects at entry. By 1 and 3 years, up to 55% and 78% of subjects, respectively, started PD medication.
Methods: Data was download from the PPMI in June 2017. We analyzed the rate of change in MDS-UPDRS total score and in DatScan striatal binding ration (SBR) prior to and after medication. Only practically-defined “off” condition data were used for visits after PD meds. Change in MDS-UPDRS was estimated from a mixed effects model for repeated measures. Demographics and baseline disease characteristics were also examined.
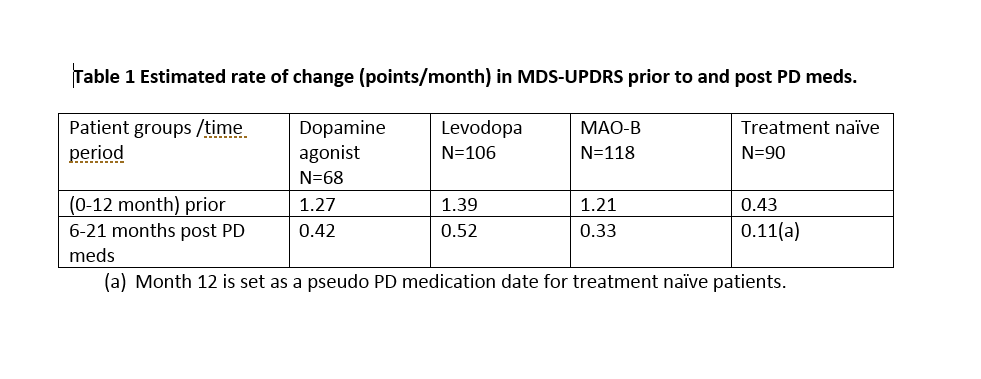
Results: As of the download date, 90 subjects remained treatment naïve; 68, 106 and 118 had begun treatment with dopamine agonists, Levodopa and MAO-B inhibitors. Baseline demographics were similar across groups; age, baseline total MDS-UPDRS scores and H&Y score were slightly higher in the Levodopa group. Table 1 shows the estimated rate of change in the MDS-UPDRS prior to and after starting PD meds. [Table 1]
Conclusions: Patients who started PD medication in PPMI had a higher rate disease progression in the year prior to starting PD medication, and levodopa-treated subjects had slightly more severe disease at study entry. The rate of change in MDS-UPDRS on medication was lower compared to before medication. Potential explanations for this observation will be discussed. There appeared to be no significant effect of medication on the rate of change of DaT SBR in any striatal region, relative to the general trend in the subjects who did not start medication in this observational study. Part of this abstract is presented at the 2018 AAN conference, April 21-28, 2018.
To cite this abstract in AMA style:
J. Cedarbaum, K. Evans, M. Yang, J. Xiao. Effect of PD medication on disease progression as measured by rate of change in MDS_UPDRS and DaT SBR in PPMI study [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/effect-of-pd-medication-on-disease-progression-as-measured-by-rate-of-change-in-mds_updrs-and-dat-sbr-in-ppmi-study/. Accessed February 2, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effect-of-pd-medication-on-disease-progression-as-measured-by-rate-of-change-in-mds_updrs-and-dat-sbr-in-ppmi-study/