Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To investigate the value of adding the dopamine agonist (DA) rotigotine (RTG) to low (<400mg/day) or high (≥400mg/day) levodopa (Ldopa) doses in patients with advanced-stage Parkinson’s disease (PD).
Background: Ldopa is the gold standard treatment for PD; however, its use is associated with the development of motor complications over time and as cumulative dose increases. Addition of a DA is a treatment strategy that may be employed to delay or minimize motor complications.
Methods: Post-hoc analysis of pooled data from patients with advanced-stage PD who received mean daily Ldopa doses of <400mg (Ldopa low dose [LD]) or ≥400mg (Ldopa high dose [HD]) combined with RTG or placebo in one of 8 randomized placebo-controlled studies (PD0004 [NCT01744496], PD0005 [NCT01782222], SP511, SP515 [NCT00244387], SP650, SP889 [NCT00474058], SP921 [NCT00522379], SP1037 [NCT01646255]) with maintenance period 4−24 weeks. Efficacy variables: change from Baseline to End of Maintenance in Unified Parkinson’s Disease Rating Scale (UPDRS), II, III, and II+III total scores, and absolute OFF time.
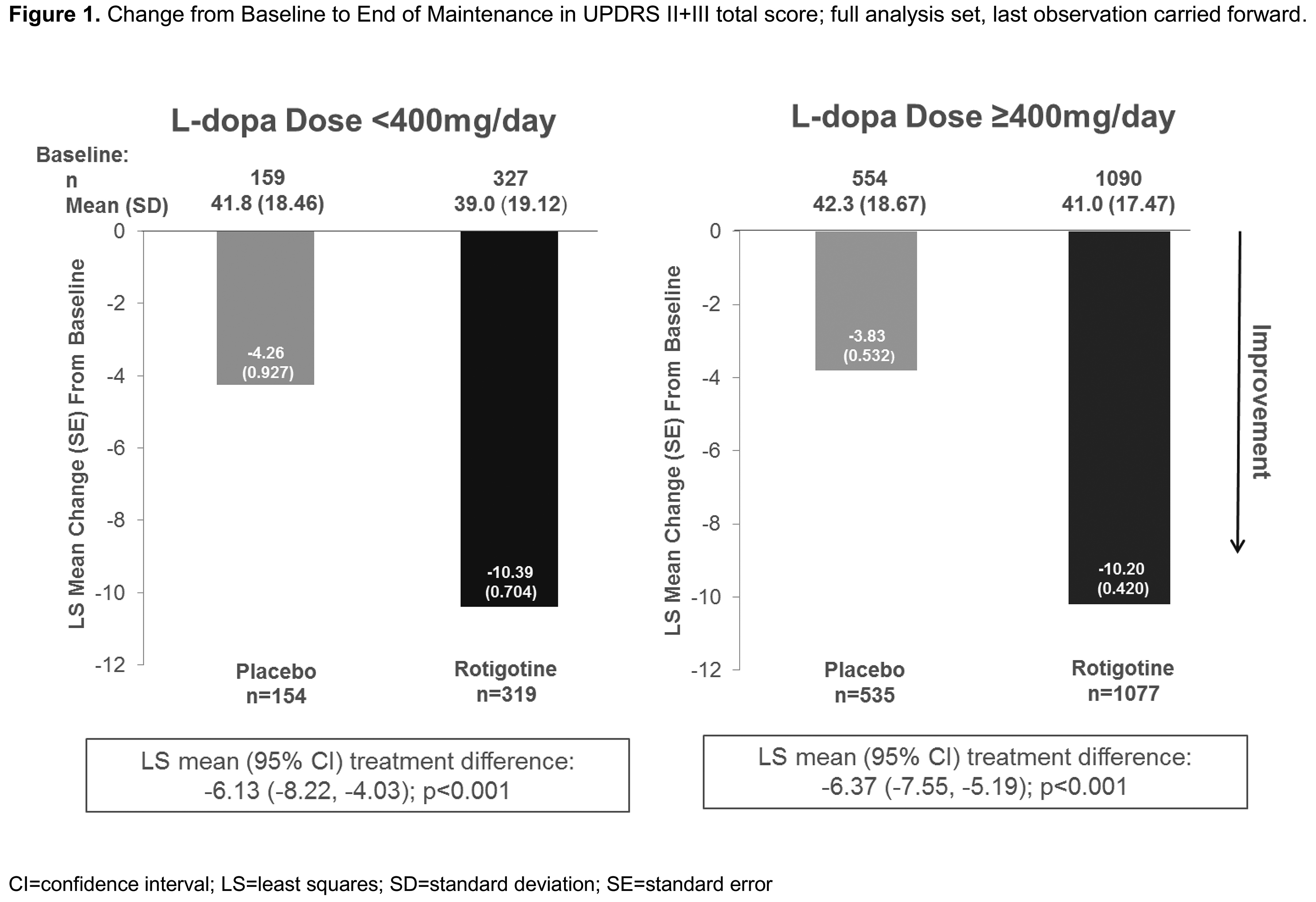
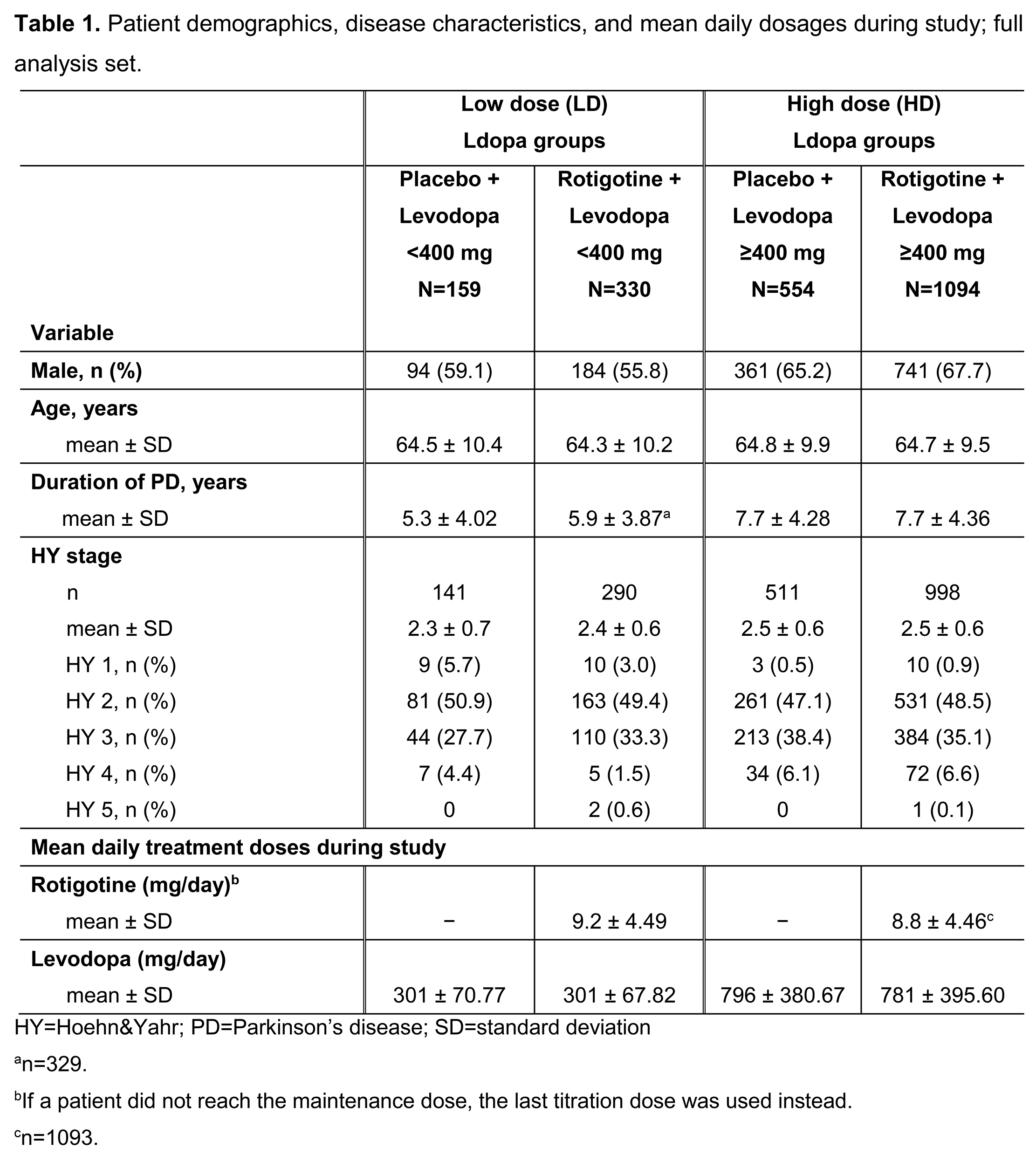
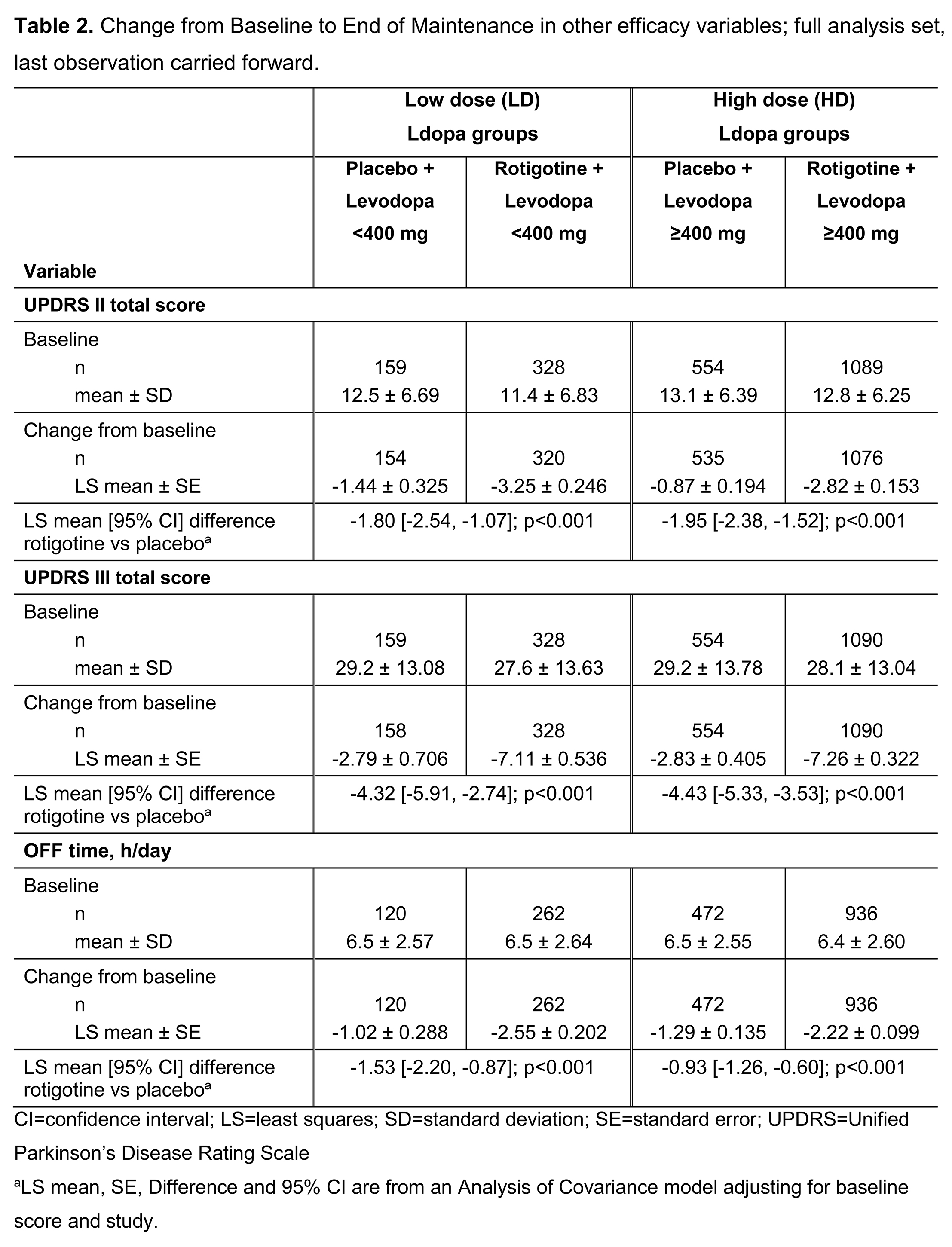
Results: Patient demographics, disease characteristics (Table 1) and efficacy (Table 2) data were available for 2137 patients (full analysis set): 489 Ldopa LD, 1648 Ldopa HD. Patients on Ldopa HD had longer disease duration vs those on Ldopa LD. Mean±SD RTG dose (mg/day) was 9.2±4.49 (Ldopa LD) vs 8.8±4.46 (Ldopa HD). Mean±SD Ldopa dose (mg/day) ranged from 301±67.82 (LD+RTG) to 796±380.67 (HD+placebo) (Table 1). RTG showed more improvement vs placebo in UPDRS II+III (Figure 1), UPDRS II, UPDRS III, and OFF time when added to either Ldopa LD or HD.
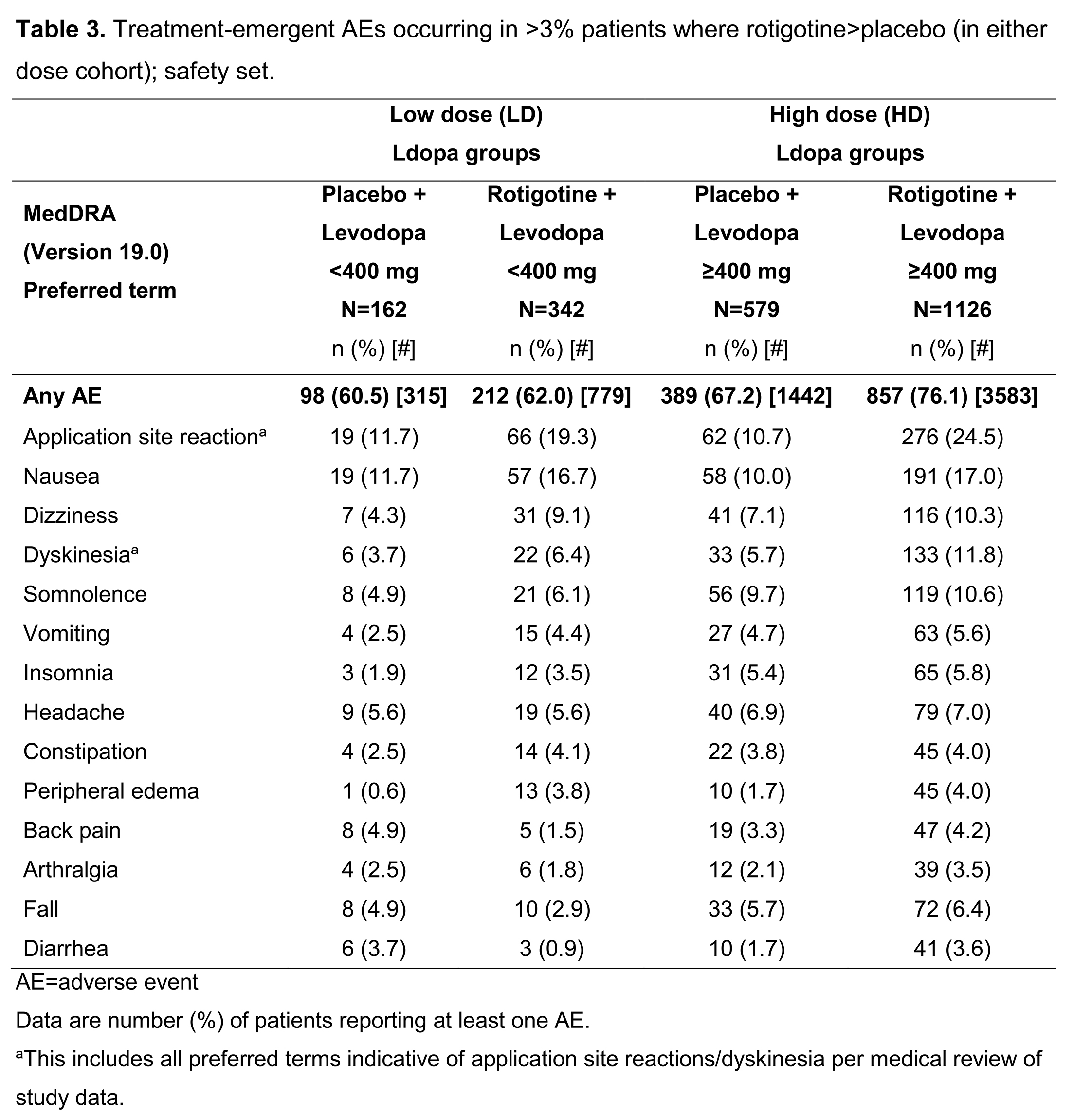
Safety data were available for 2209 patients (safety set): 504 Ldopa LD, 1705 Ldopa HD. Overall, adverse events (AEs) were more common with HD Ldopa; the most common AEs in both dose groups (where RTG>placebo) were application site reactions, nausea, dizziness, and dyskinesia (Table 3).
Conclusions: RTG improved UPDRS II+III, II, and III total scores, and absolute OFF time vs placebo, whether added to low (<400mg/day) or high (≥400mg/day) dose Ldopa. This suggests physicians may consider adding RTG to low dose Ldopa when PD patients need additional symptomatic control, rather than increasing Ldopa dose, potentially delaying worsening of motor complications and maximizing patients’ long-term treatment options.
Funding: UCB Pharma.
References: [table1]
[table2]
[figure1]
[table3]
To cite this abstract in AMA style:
E. Dohin, F. Woltering, M. Asgharnejad. Evaluation of rotigotine transdermal patch in combination with low or high dose levodopa in patients with Parkinson’s disease: post-hoc analysis [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/evaluation-of-rotigotine-transdermal-patch-in-combination-with-low-or-high-dose-levodopa-in-patients-with-parkinsons-disease-post-hoc-analysis/. Accessed February 21, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluation-of-rotigotine-transdermal-patch-in-combination-with-low-or-high-dose-levodopa-in-patients-with-parkinsons-disease-post-hoc-analysis/