Session Information
Date: Thursday, June 8, 2017
Session Title: Dystonia
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To explore minimal clinically important change perceived by patients as beneficial by the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) using data from the Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy (CD PROBE) study.
Background: Although ≥10-point or ≥30% difference has been reported for defining clinically important change, there is no validated definition of clinically meaningful response to onabotulinumtoxinA treatment for CD.
Methods: CD PROBE is a multicenter, prospective clinical registry that recruited CD patients from 88 US sites. CD patients who were new to botulinum toxin therapy or had not received it for ≥16 weeks and were deemed suitable for onabotulinumtoxinA received 3 cycles of drug. Changes from baseline in TWSTRS scores were correlated with clinical improvement as assessed by the Patient or Clinical Global Impression of Change (PGIC/CGIC) scales. The model was discriminated using logistic regression; good discrimination was defined as ≥70% correct classification of PGIC or CGIC based on TWSTRS scores. We report on the 479 patients with TWSTRS scores who completed the study.
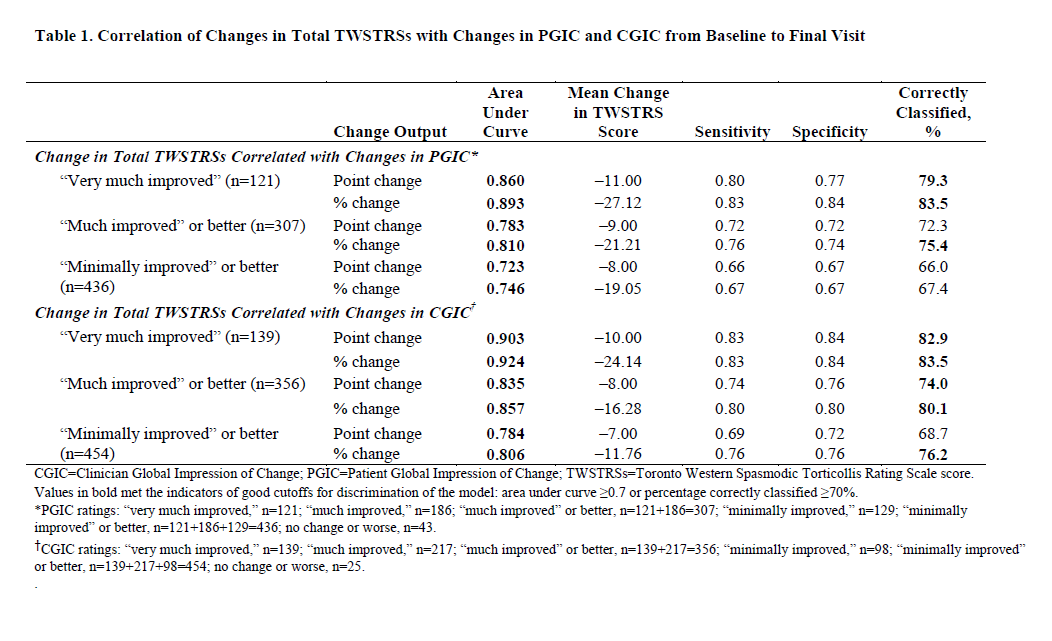
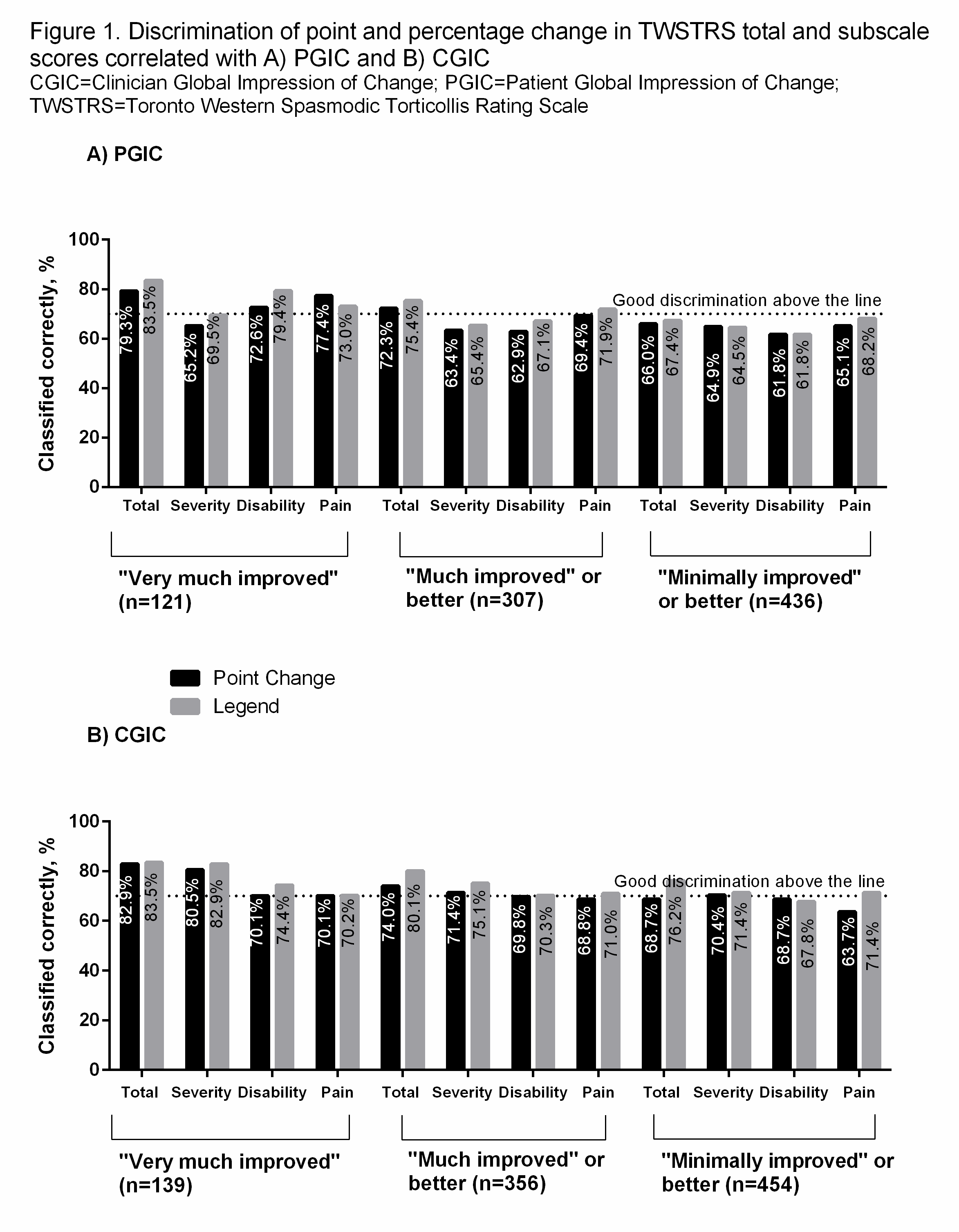
Results: Mean TWSTRS score significantly decreased from baseline to final visit (39.2 to 27.1, P<0.0001). Point (percentage) reduction in total TWSTRS score correlating with “very much improved,” “much improved” or better, and “minimally improved” or better on the PGIC were 11 (27.1%, n=121), 9 (21.2%, n=307), and 8 (19.1%, n=436), respectively; for CGIC were 10 (24.1%, n=139), 8 (16.3%, n=356), and 7 (11.8%, n=454) [table1]. Total TWSTRS score had adequate discrimination and correlated with PGIC and CGIC better than any TWSTRS subscale scores [figure 1]. The small group sizes for the “minimally improved” group (PGIC, n=129; CGIC, n=98) limited the ability to fully determine the clinical relevance of the correlation of PGIC and CGIC with total TWSTRS scores.
Conclusions: Change in total TWSTRS score correlated well with patient- and clinician-based evaluations (PGIC and CGIC, respectively) of onabotulinumtoxinA-treated patients with CD. These results approximate definitions of response used in other studies.1,2
Data previously submitted to American Academy of Neurology 2017 Annual Meeting, and accepted by Conference of the International Neurotoxin Association, 2017
References: 1. Truong D, et al. Mov Disord. 2005;20(7):783-791.
2. Comella CL, et al. J Neurol Sci. 2011;308(1-2):103-109.
To cite this abstract in AMA style:
K. Dashtipour, Z. Mari, J. Jankovic, C. Adler, M. Schwartz, M. Brin. Clinical Relevance of Treatment With OnabotulinumtoxinA in Patients With Cervical Dystonia: Results From the CD Probe Study [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/clinical-relevance-of-treatment-with-onabotulinumtoxina-in-patients-with-cervical-dystonia-results-from-the-cd-probe-study/. Accessed November 8, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-relevance-of-treatment-with-onabotulinumtoxina-in-patients-with-cervical-dystonia-results-from-the-cd-probe-study/