Session Information
Date: Wednesday, June 7, 2017
Session Title: Parkinson's Disease: Genetics
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
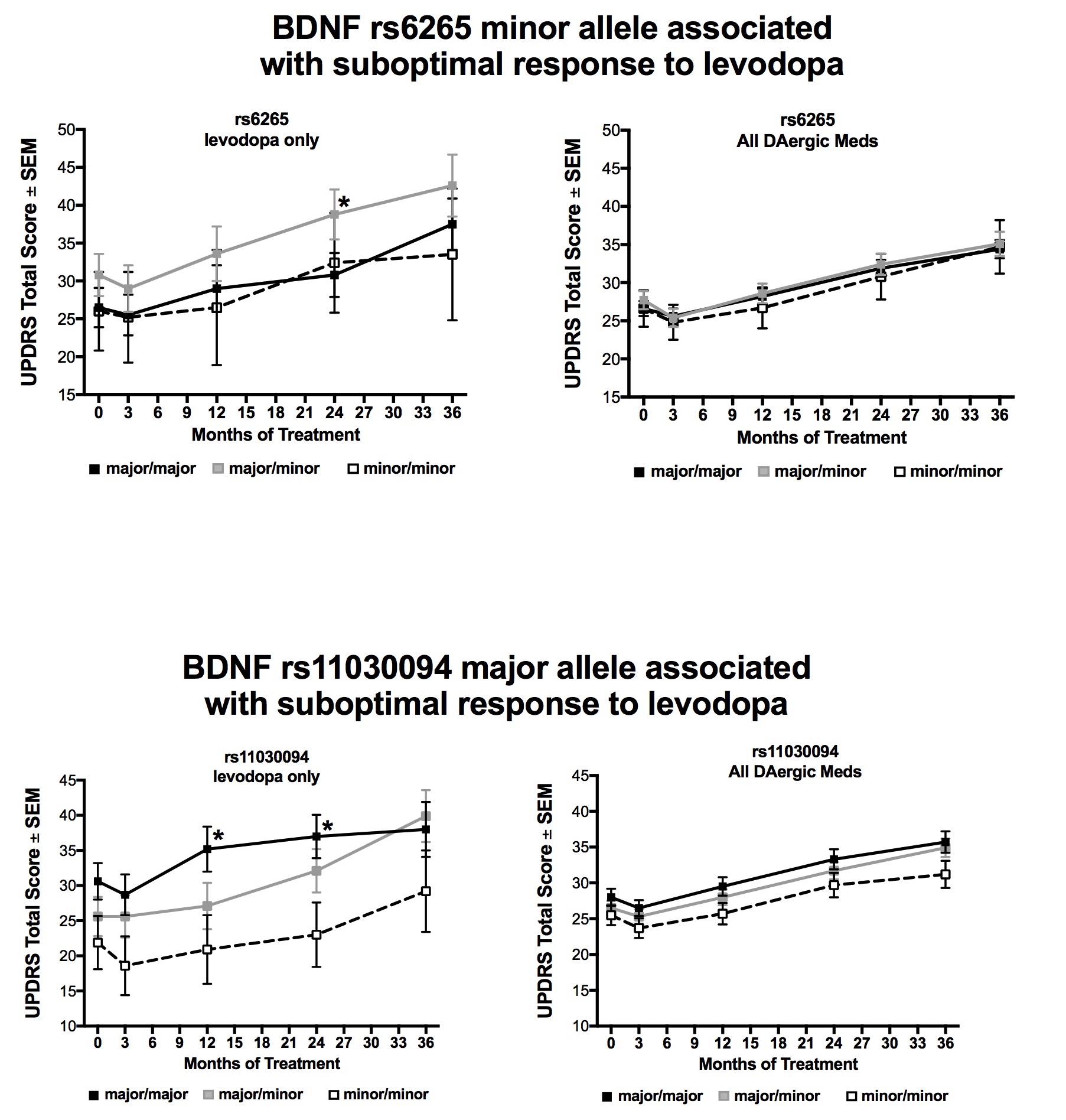
Objective: We examined the impact of rs6265 and other brain-derived neurotrophic factor (Bdnf) variants in two subject cohorts: 1) early-stage Parkinson’s disease (PD) subjects from the NIH NINDS Exploratory Trials in PD Long-term Study-1 (NET-PD LS-1) treated with either levodopa alone (l-dopa, n=56) or any combination of dopaminergic medications (DA meds, n=540), and 2) 15 mid/late-stage PD subjects treated with subthalamic nucleus deep brain stimulation (STN DBS).
Background: The ability to use patient genotype to design optimal antiparkinsonian treatment strategies would be a powerful approach. BDNF is a critical modulator of neurotransmission and plasticity in the basal ganglia. Preclinical work has implicated BDNF signaling in the antiparkinsonian efficacy of both levodopa and deep brain stimulation (DBS). Several single nucleotide variants exist in the Bdnf gene, one in particular (rs6265) results in reduced activity-dependent BDNF release. Previously we showed that early-stage PD subjects carrying the rs6265 minor allele exhibit a less robust response to l-dopa therapy, whereas rs6265 variant status did not alter patient response to STN DBS.
Methods: Subjects were genotyped for six Bdnf variants, including rs6265. Response to therapy was assessed by UPDRS scores at various visits over 36 months. Mean UPDRS scores in NET-PD LS-1 were stratified by variant allele status and adjusted for site, age, race, PD duration, therapy dose, and treatment group.
Results: In l-dopa treated subjects, rs6265 minor allele carriers and rs11030094 major allele carriers exhibited higher mean total UPDRS scores compared to subjects without the risk allele at 24 months (rs6265: 37.6 vs. 30.7, p=0.04; re11030094: 35.0 vs. 23.8, p=0.01 compared to minor/minor). Neither allele was associated with an altered response to DA meds or STN DBS. Possession of multiple BDNF variant risk alleles was associated with a significantly worse therapeutic response to l-dopa (mean total UPDRS at 24 months for 0-1, 2-3, 3-6 risk alleles=21.0 , 31.7, 38.8, respectively, p=0.001), but not DA meds.
Conclusions: Two of the six risk alleles for Bdnf variants were associated with a worsened therapeutic response to levodopa. Genotyping for specific Bdnf variants may be a useful precision medicine approach to identify optimal antiparkinsonian treatment strategies.
To cite this abstract in AMA style:
C. Sortwell, P. Auinger, J. Goudreau, B. Pickut, B. Berryhill, M. Hacker, D. [email protected], J. Lipton, A. Cole-Strauss, J. Elm, D. Fischer. Specific Bdnf variants are associated with suboptimal response to levodopa but not to other dopaminergic medications or deep brain stimulation in Parkinson’s disease [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/specific-bdnf-variants-are-associated-with-suboptimal-response-to-levodopa-but-not-to-other-dopaminergic-medications-or-deep-brain-stimulation-in-parkinsons-disease/. Accessed January 1, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/specific-bdnf-variants-are-associated-with-suboptimal-response-to-levodopa-but-not-to-other-dopaminergic-medications-or-deep-brain-stimulation-in-parkinsons-disease/