Session Information
Date: Wednesday, June 7, 2017
Session Title: Parkinson's Disease: Cognition
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To determine the feasibility of utilizing the NIH Toolbox in moderate to advanced disease Parkinson’s disease (PD) subjects with bilateral subthalamic nucleus deep brain stimulation (STN-DBS).
Background: The CAPSIT-PD protocol recommends a series of neuropsychological measures which have poor tolerability in PD patients undergoing DBS [1]. A novel assessment tool, the NIH Toolbox cognition battery [2], has been developed to serve as a brief, convenient set of measures that provides a “common currency” among researchers for comparisons across a wide range of studies and populations. Though the NIH Toolbox was released in 2012 and is listed as one of the “common data elements,” it’s use has only been described in PD patients with mild disease and it has not been utilized in the DBS population.
Methods: We enrolled 14 PD subjects with bilateral STN-DBS who were administered the NIH Toolbox cognition battery in the ON-medication, ON-stimulation state. Additional data collected included basic demographic information, Montreal Cognitive Assessment (MoCA), and Unified Parkinson’s disease Rating Scale (UPDRS). The NIH Toolbox battery fully-corrected t-scores were analyzed which have a normative mean of 50 with an SD of 10, and are corrected for age, education, sex, and race.
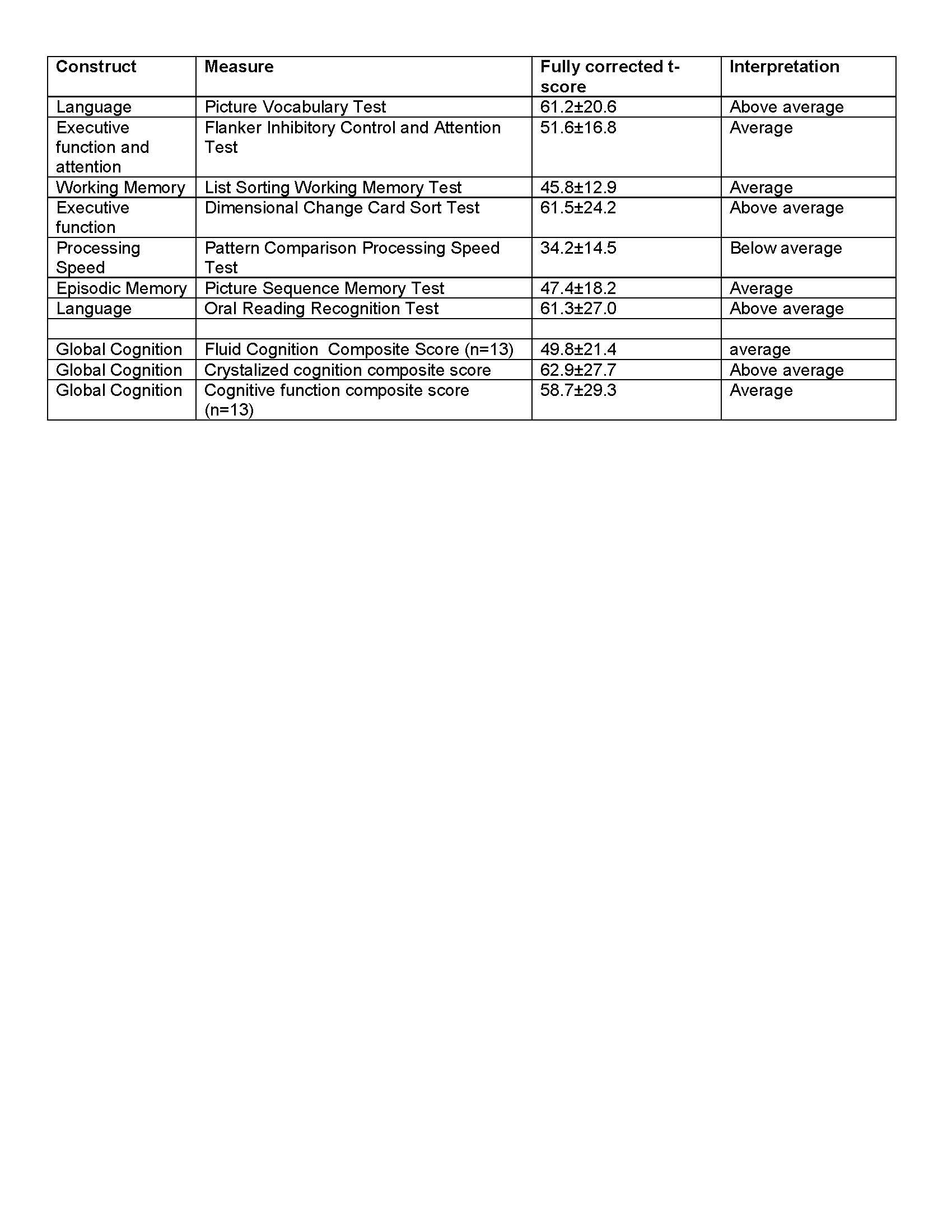
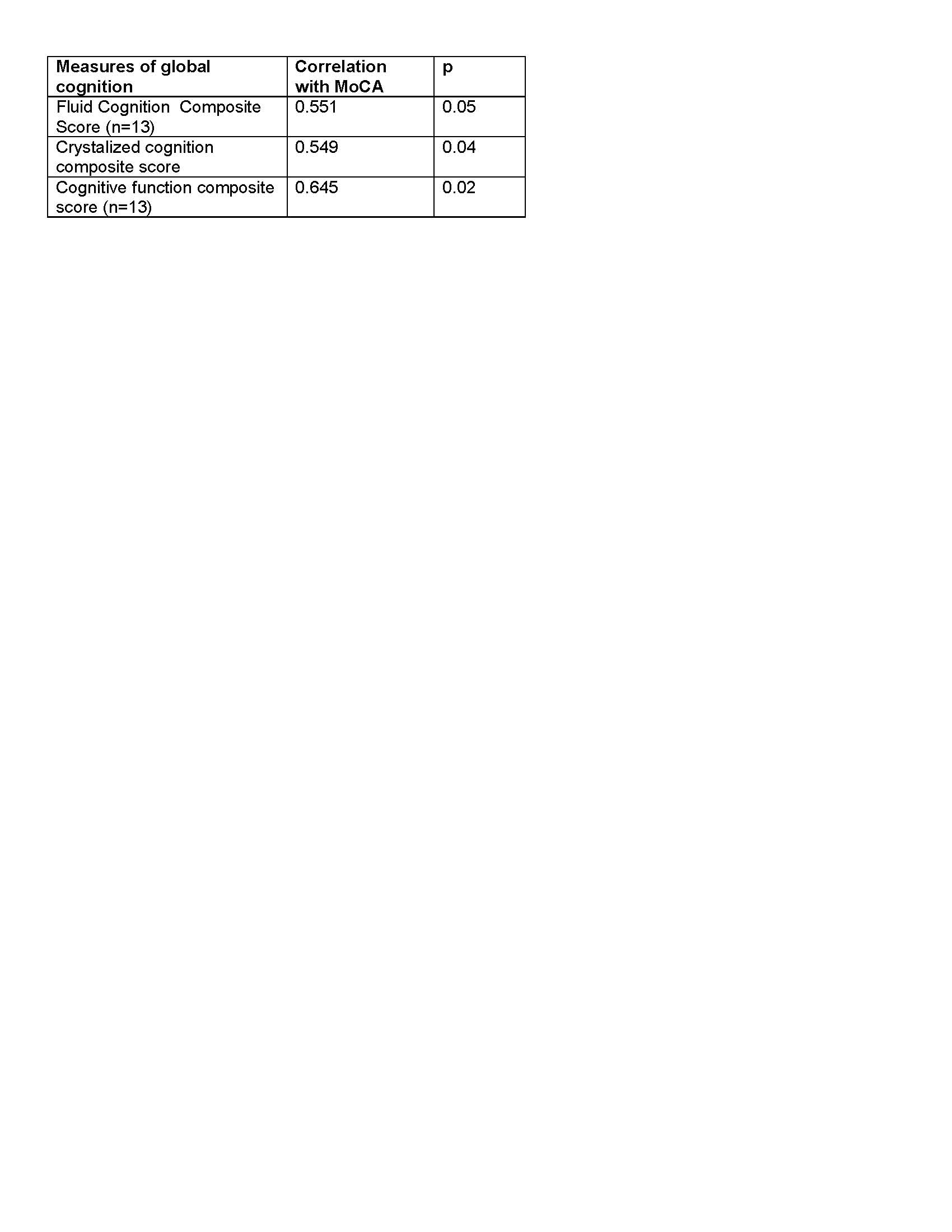
Results: Fourteen PD subjects were enrolled with a mean age of 63.9±6.5, mean disease duration of 12.8±7.1 years, and 78.6 percent were men. All subjects had bilateral STN-DBS with a mean of 1.7±1.1 years of DBS duration. Mean UPDRS-III score was 16.6±8.2 years and mean MoCA score was 24.9±5.1. All but one subject (13/14) completed the full range of cognitive measures. Subjects performed in the average to above average range in nearly all measures except for processing speed where individuals performed significantly below average. Overall cognitive function was in the average to above average level based on the toolbox composite scores [table 1]. There was a moderate correlation between the NIH Toolbox global cognitive measures and MoCA scores, with the cognitive function composite score having the strongest correlation with MoCA [table 2].
Conclusions: The NIH Toolbox cognition battery is feasible to administer to moderate to advanced PD patients with bilateral STN-DBS and has modest correlation with the MoCA, a widely used assessment tool in PD. Further studies are needed to validate the NIH Toolbox in the PD population.
References: [1] Pal, G. D., Persinger, V., Bernard, B., Ouyang, B., Goetz, C. G. and Verhagen Metman, L. (2015), The Core Assessment Program for Surgical Interventional Therapies in Parkinson’s Disease (CAPSIT-PD): Tolerability of Preoperative Neuropsychological Testing for Deep Brain Stimulation in Parkinson’s Disease. Mov Disord Clin Pract, 2: 379–383.
[2] Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013 Mar 12;80(11 Suppl 3):S2-6.
To cite this abstract in AMA style:
J. Emerson, P. Dhruva, C. McLeod, R. Moses-Kessler, L. Metman, G. Pal. Feasibility of utilizing the NIH Toolbox in Parkinson’s disease subjects with deep brain stimulation [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/feasibility-of-utilizing-the-nih-toolbox-in-parkinsons-disease-subjects-with-deep-brain-stimulation/. Accessed January 1, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/feasibility-of-utilizing-the-nih-toolbox-in-parkinsons-disease-subjects-with-deep-brain-stimulation/