Session Information
Date: Tuesday, June 6, 2017
Session Title: Rare Genetic and Metabolic Diseases
Session Time: 1:45pm-3:15pm
Location: Exhibit Hall C
Objective: To evaluate the efficacy and safety of chelation therapy with deferiprone combined with phlebotomy in reducing iron stores and neurological progression in aceruloplasminemia.
Background: Aceruloplasminemia is a rare form of Neurodegeneration with Brain Iron Accumulation (NBIA). No treatments have yet been reported giving sustained clinical benefit at a neurologically symptomatic stage of the disease (1). Deferiprone is the only chelator available which might act beyond the blood brain barrier, and is capable of redistributing iron to hematopoietic tissues by transferring it to transferrin. Phlebotomy is a more effective iron remover than deferiprone alone. The combination of deferiprone and phlebotomy has not yet been reported.
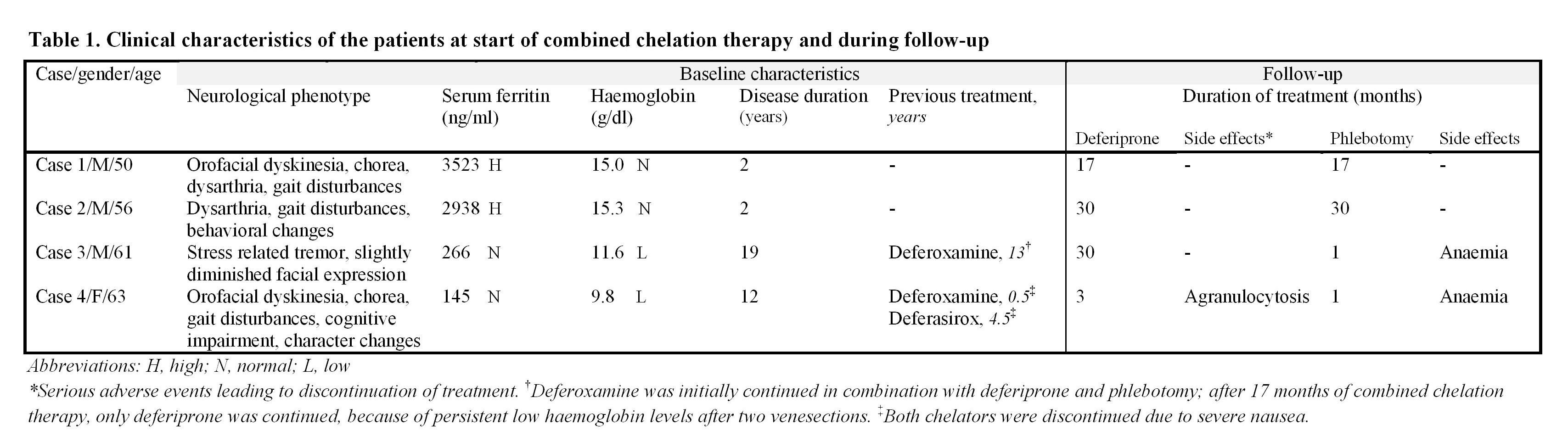
Methods: Four Dutch patients with homozygosity for G631R in the CP gene (2) received deferiprone to a maximum dose of 65 mg/kg/day in combination with phlebotomies of up to 500 ml blood/2 weeks. Safety and tolerability were monitored. Neurological function was quantitatively assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS III-Motor Section) and the Scale for the Assessment and Rating of Ataxia (SARA), every 3 months for the first year of treatment and every 6 months thereafter. Systemic iron stores were measured by ferritin levels and brain MRI was performed yearly to evaluate cerebral iron deposition.
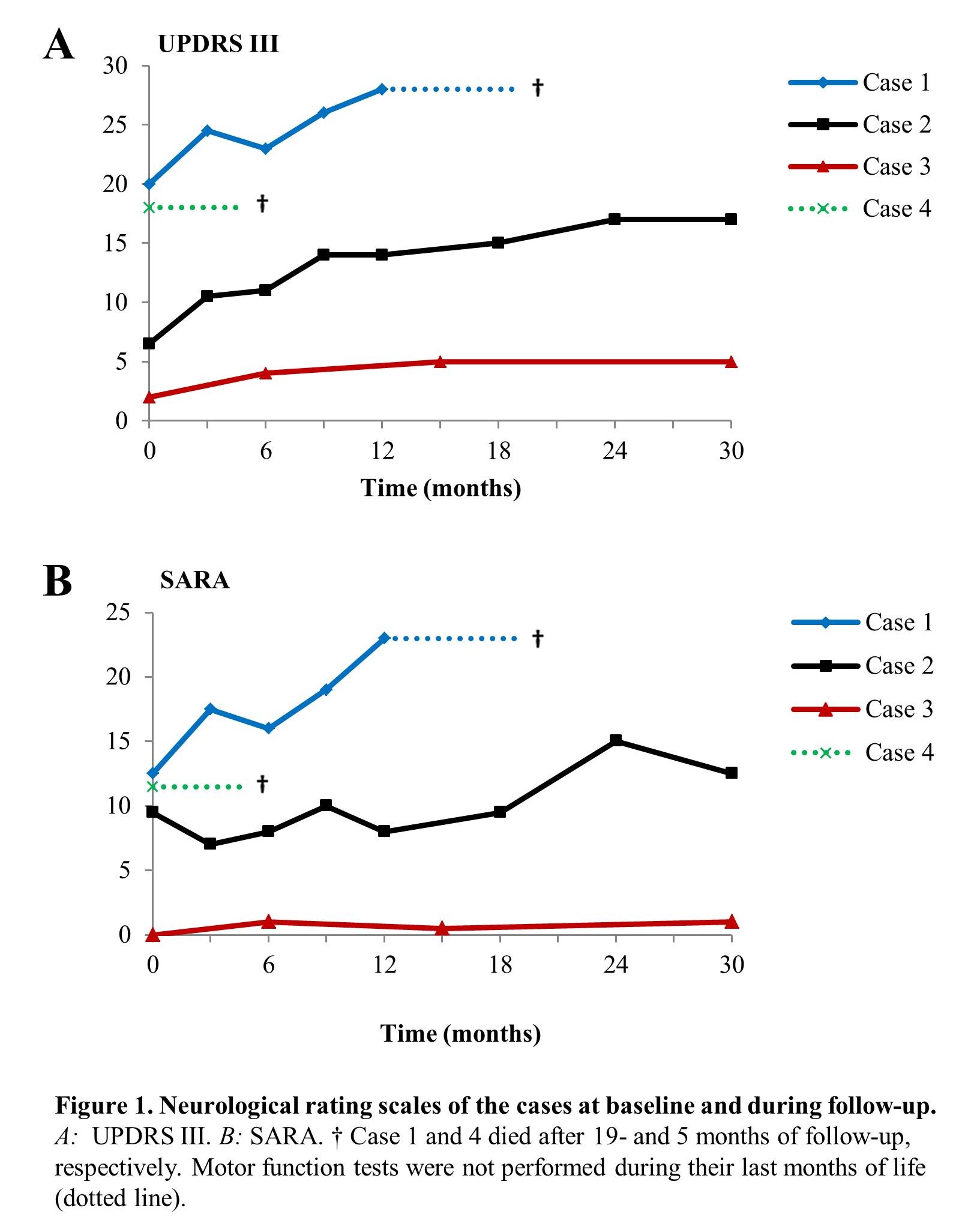
Results: Deferiprone combined with phlebotomy was well tolerated in case 1 and 2; in the other cases the treatment regimen was early discontinued [table 1]. After 30 months of treatment motor features had gradually worsened in case 2; case 3 showed stable neurological disease. Case 1 and 4 died during follow-up [figure 1]. Ferritin levels decreased from 3523- to 113 ug/l and 2938- to 605 ug/l in case 1 and 2, respectively; in case 3 and 4 serum ferritin remained normal. Conventional MRI of the brain showed no obvious changes in iron distribution in case 2 and 3; in case 1 progressive iron accumulation was found.
Conclusions: Deferiprone combined with phlebotomy proved to be safe and effective in reducing serum ferritin in two non-anaemic aceruloplasminemia patients. Phlebotomy should not be tried in combination with deferoxamine or in the presence of anaemia. The progression of neurological disease despite biochemical effectiveness in patients with advanced manifestations underlines the importance of early diagnosis and prompt initiation of treatment.
References:
- Dusek P, Schneider SA, Aaseth J. Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol 2016;38:81-92.
- Vroegindeweij LH, Langendonk JG, Langeveld M, et al. New insights in the neurological phenotype of aceruloplasminemia in Caucasian patients. Parkinsonism Relat Disord 2016. doi: 10.1016/j.parkreldis.2016.12.010
To cite this abstract in AMA style:
L.H.P. Vroegindeweij, J.G. Langendonk, J.H.P. Wilson, A.J.W. Boon. Deferiprone combined with phlebotomy for aceruloplasminemia [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/deferiprone-combined-with-phlebotomy-for-aceruloplasminemia/. Accessed December 12, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/deferiprone-combined-with-phlebotomy-for-aceruloplasminemia/