Session Information
Date: Monday, June 5, 2017
Session Title: Surgical Therapy: Parkinson’s Disease
Session Time: 1:45pm-3:15pm
Location: Exhibit Hall C
Objective: Evaluation of clinically optimized settings in bilateral STN-DBS Parkinson’s disease, allows anatomical correlations with benefits and side effects.
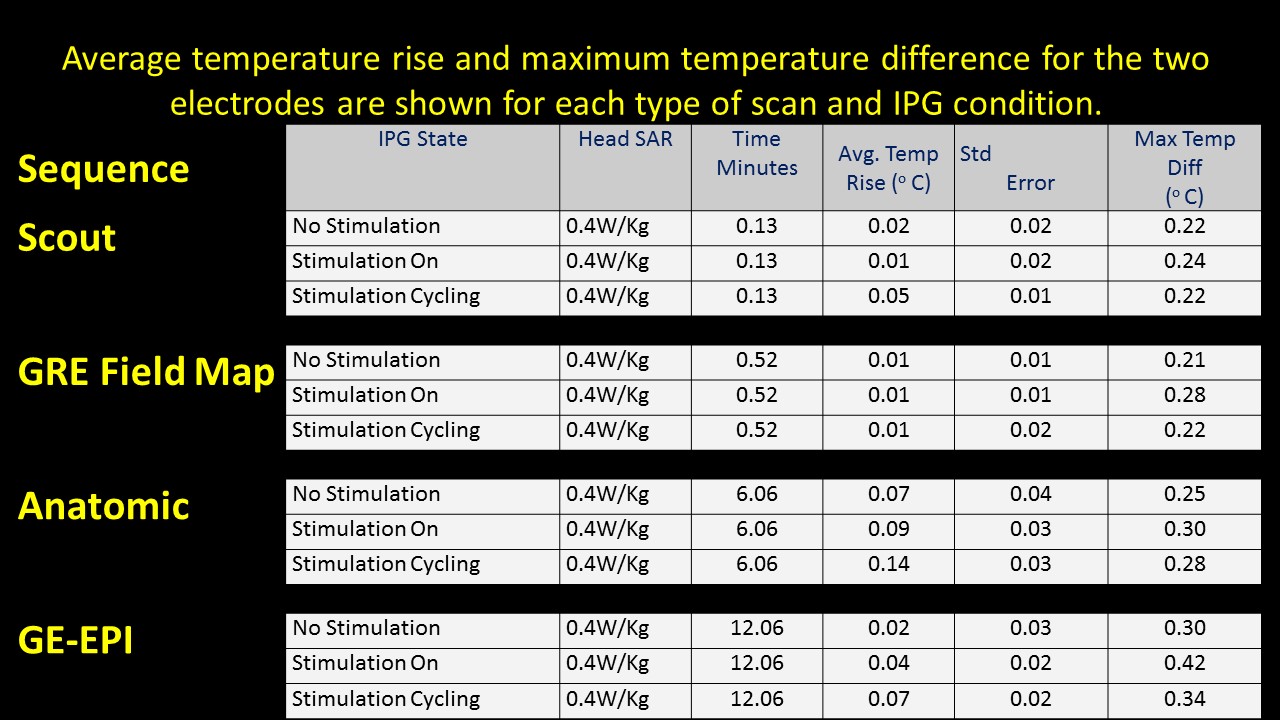
Background: MRI acquisition can cause permanent damage to implantable pulse generator (IPG) or heating of DBS leads limiting clinician’s needs to review the effects of STN-DBS on functioning of basal ganglia. A preliminary study using a phantom with Medtronic DBS leads (3389) and IPG (Activa PC 37601) conducted at University Hospitals, Cleveland, (OH), USA, determined DBS safety within MR environment. Our study is novel in two ways: (1) Use of actual IPG and electrodes in a PD patient. (2) evaluation of fMRI activation recorded in response to therapeutic and sub-therapeutic settings of IPG. We ensured (a) temperatures ≤ 1°C at the active electrode lead contacts (b) intact lead contacts (DBS impedance studies), (c) regular IPG cycles within the scanner for low SAR sequences used, and using (d) custom built Tx/Rx Head coils (e) chest implanted newer IPG with built in safety shunt circuitry (without magnetic reed switch).
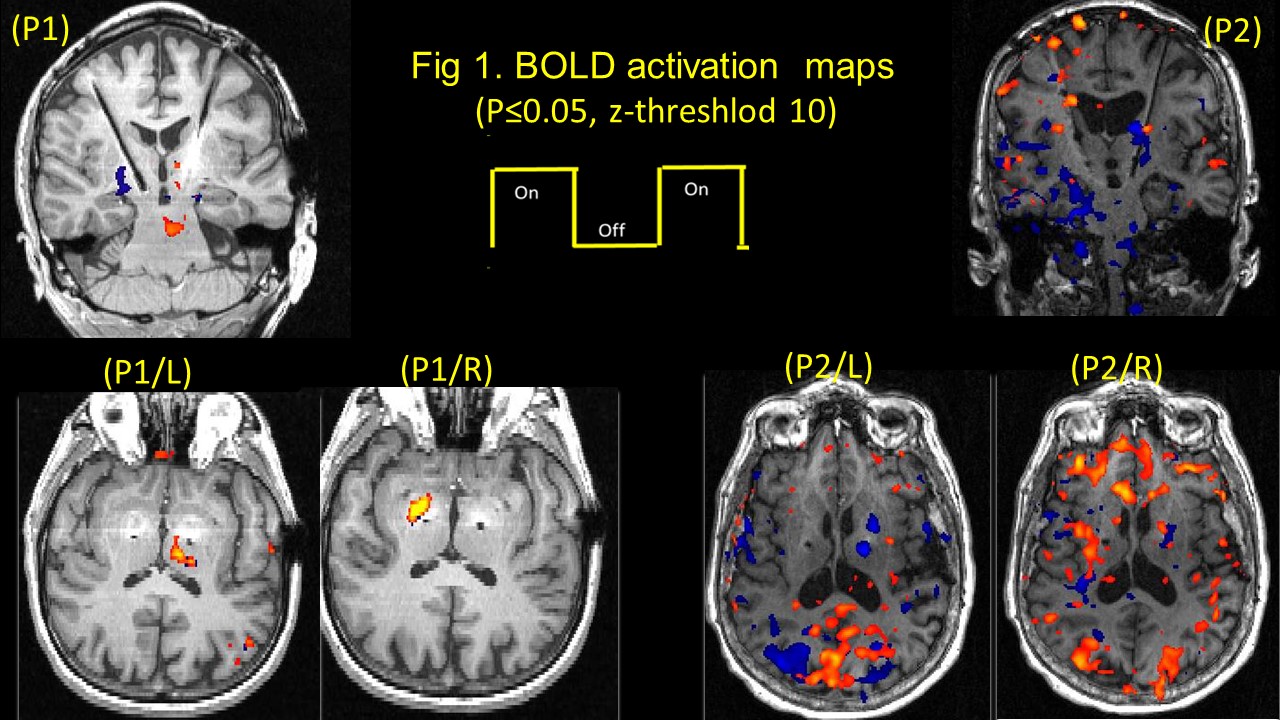
Methods: Two bilateral STN-DBS right-handed implanted PD (P1 & P2), were observed at their individual therapeutic and sub-therapeutic DBS settings. Imaging was performed (on-med) on a 3T Verio (Siemens). Sagittal T1 and EPI Images for both left and right electrode (therapeutic and sub-therapeutic DBS settings) were acquired separately with IPG cycling (30s on/off) . Data was processed using FSL. A boxcar design with six cycles of alternate DBS ‘on’ & ‘off’ was used to determine fMRI activation due to the stimulation [figure1]
Results: Therapeutically, both patients evoked BOLD activation in bilateral occipital cortex, left thalamus, and contralateral cerebellum, for the left, both patients presented a clear significant BOLD activation in the pallidum, (P1-right; P2-Left) for electrode stimulation; while sub-therapeutically, P1 presented similar activation as therapeutic for the right side, but no thalamic or pallidum activation in P2 for either sides. [figure2]
Conclusions: This study clearly indicates a safe fMRI acquisition in STN-DBS implanted patients. It opens a new direction for evaluation of patient specific therapeutic voltage threshold, apart from establishing erroneous activation responsible for side effects in STN-DBS implanted patients.
References: Phillips et al. (2006). Radiology 239:209-216.
Carmichael et al. (2007). NeuroImage 37:508-517.
To cite this abstract in AMA style:
M. Saxena, C. McIntyre, B. Walter. Safe Functional MR Imaging in STN-DBS implanted Parkinson’s disease [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/safe-functional-mr-imaging-in-stn-dbs-implanted-parkinsons-disease/. Accessed January 2, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safe-functional-mr-imaging-in-stn-dbs-implanted-parkinsons-disease/