Session Information
Date: Monday, June 5, 2017
Session Title: Surgical Therapy: Other Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Exhibit Hall C
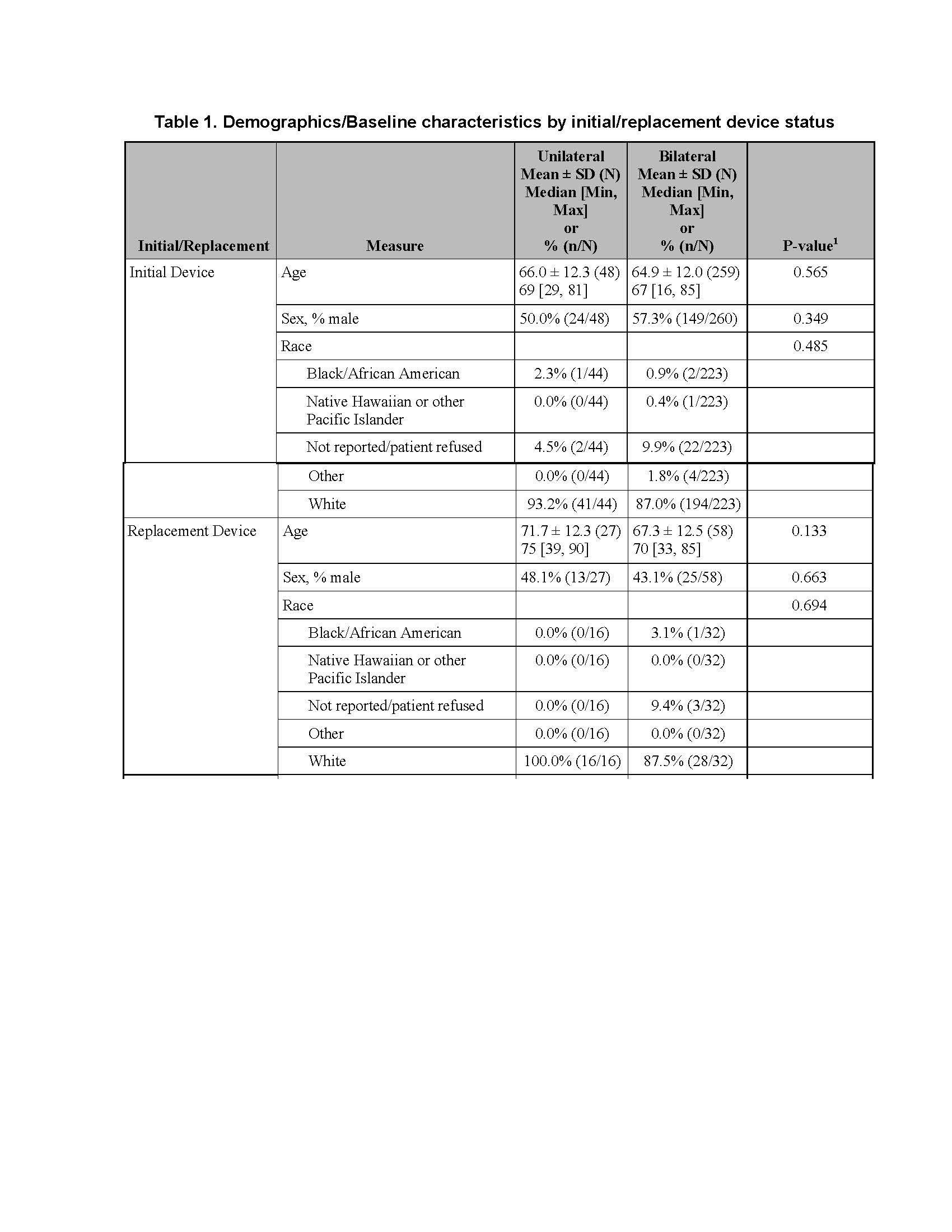
Objective: To compare the safety of unilateral (Uni) to bilateral (Blt) DBS implants in Essential Tremor (ET).
Background: DBS is an effective therapy in reducing contralateral distal hand tremor in patients with ET 1. Published literature from single sites report Blt DBS implants are associated with higher rates of dysarthria, gait or balance disorders2. Our study is based on data from an international multi-center Product Surveillance Registry (PSR, Medtronic).
Methods: Baseline information and device/implant data collected from ET patients enrolled in PSR, and followed prospectively for events related to device, implant procedure, stimulation therapy; included 394 with 76 Uni (48 initial/27 replacement (rep)/1 unspecified), and 318 simultaneous Blt lead implants (260 initial/58 rep) from 39 centers from 2009-2016. Statistical comparisons used two-sample t-test or Fisher’s for categorical measures.
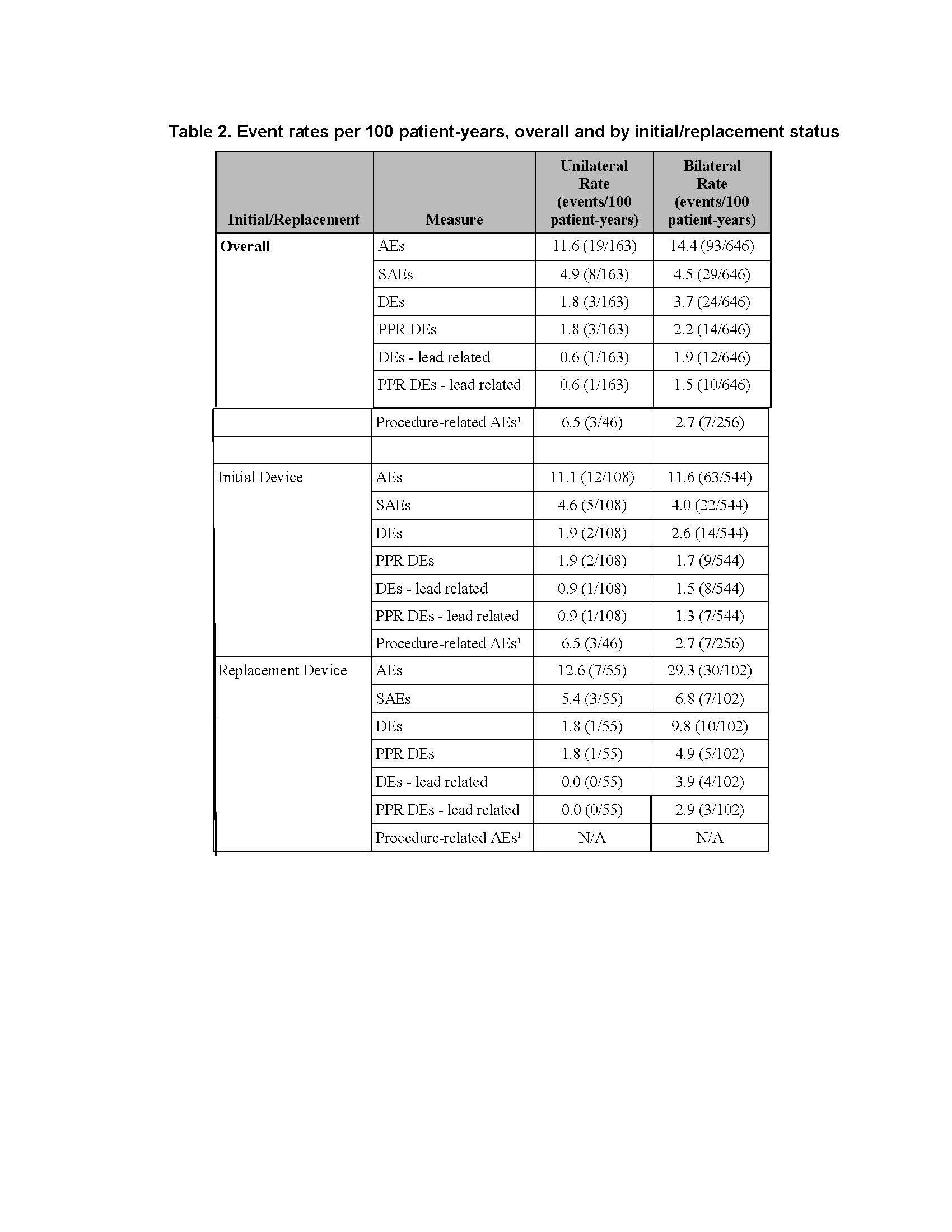
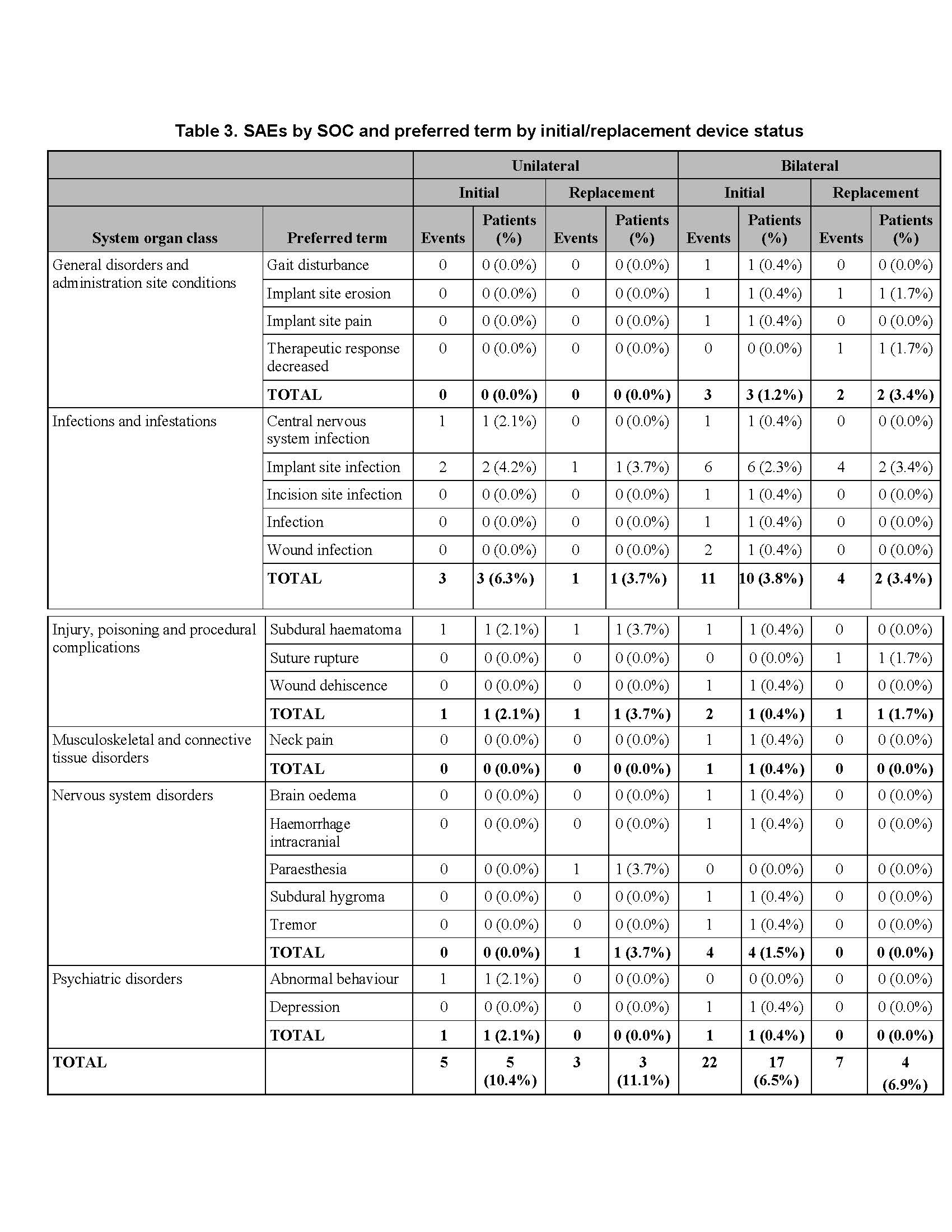
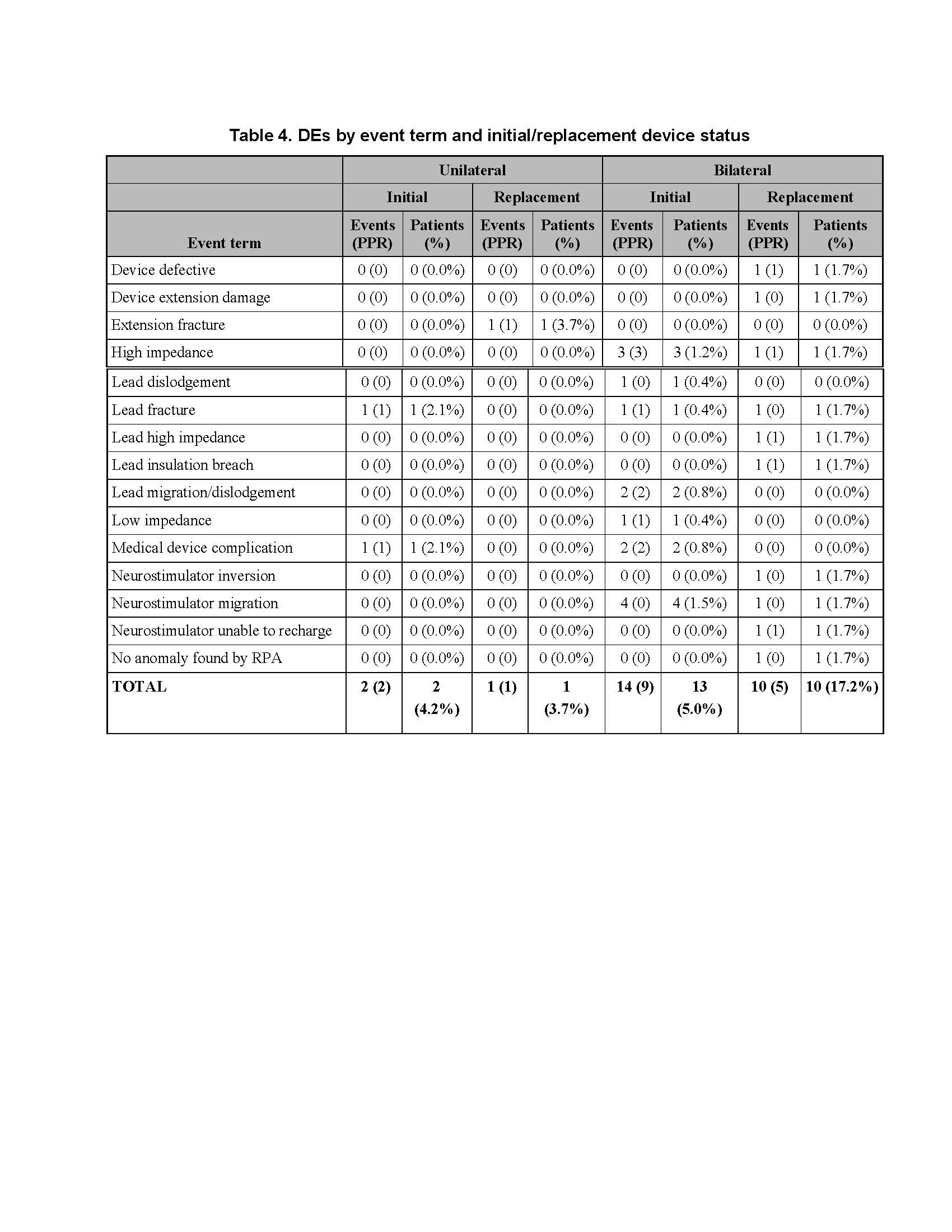
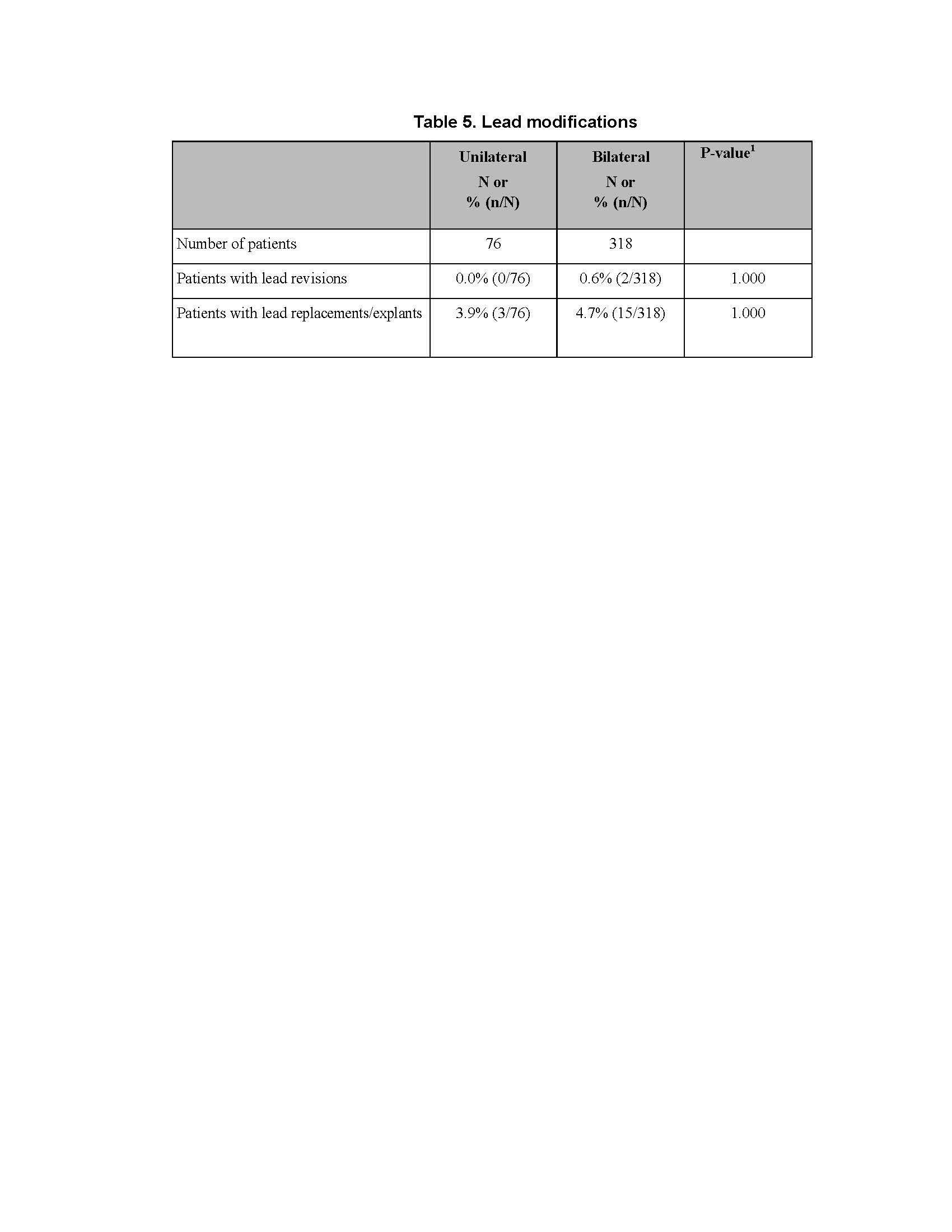
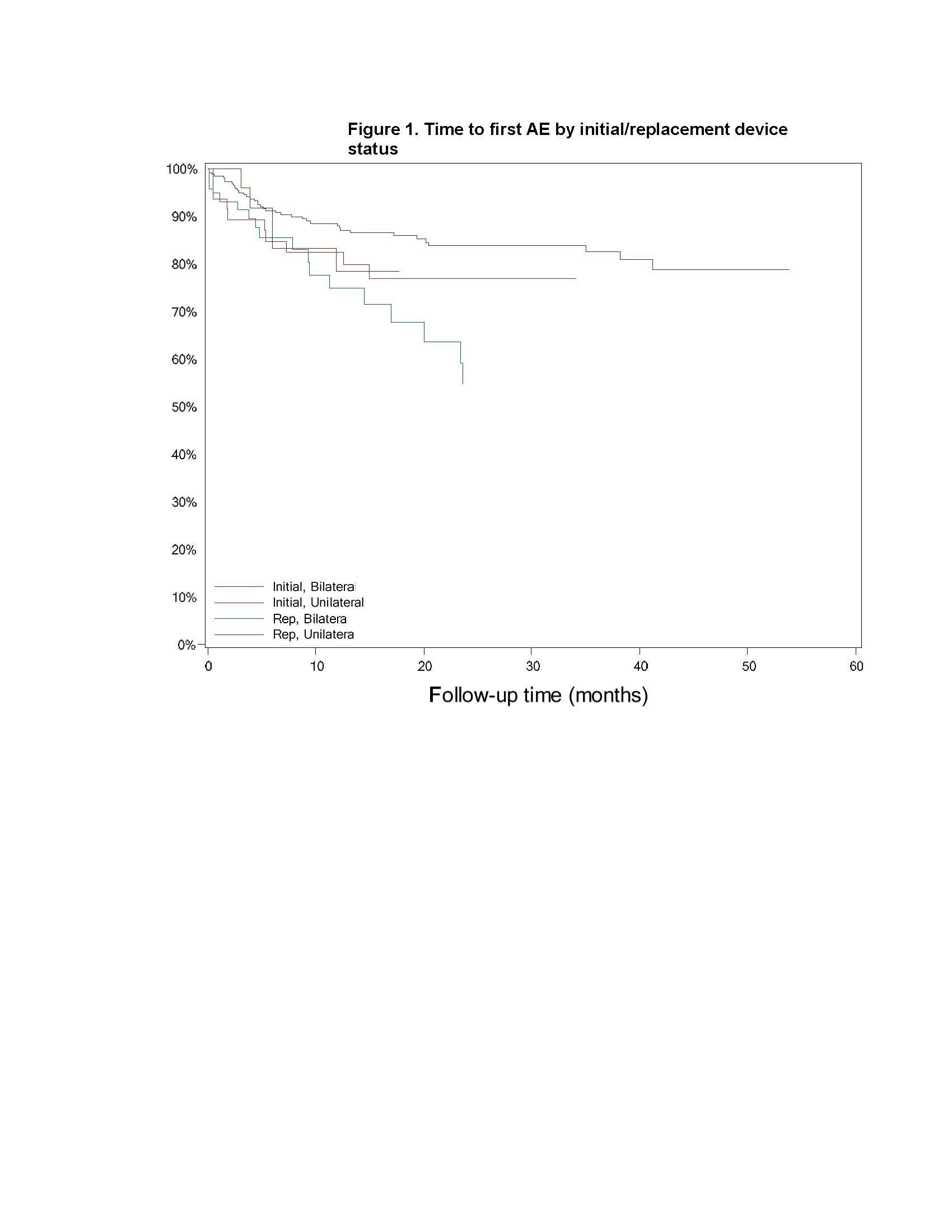
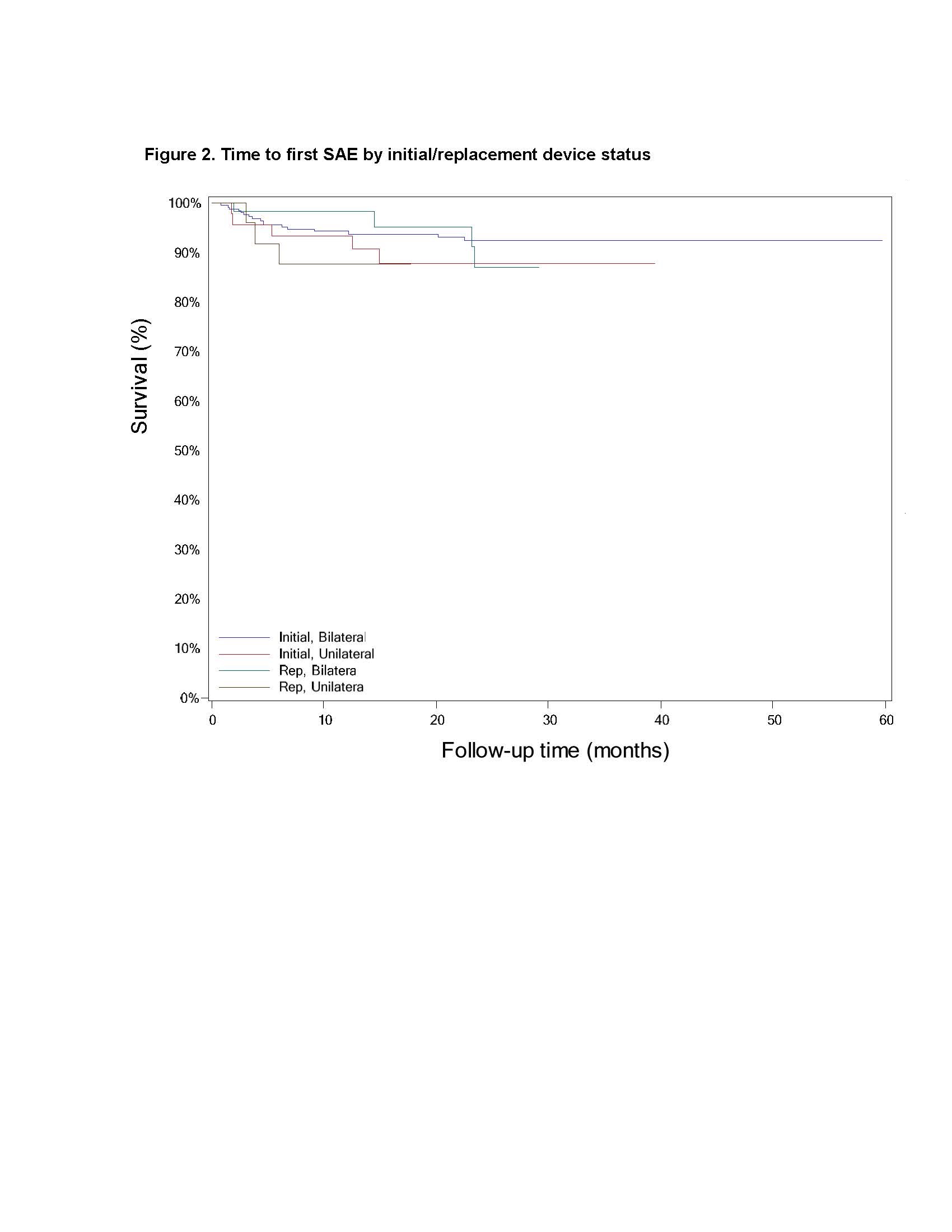
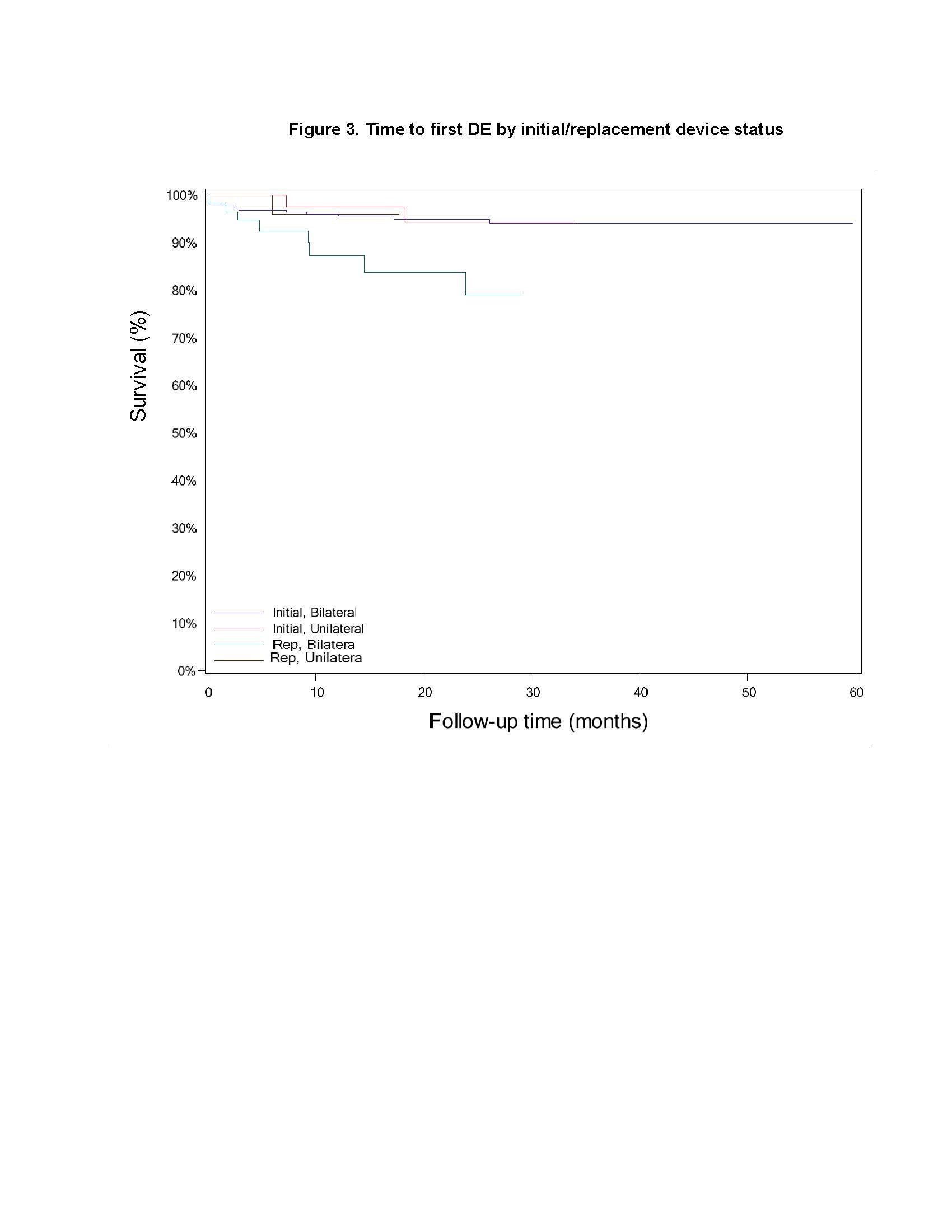
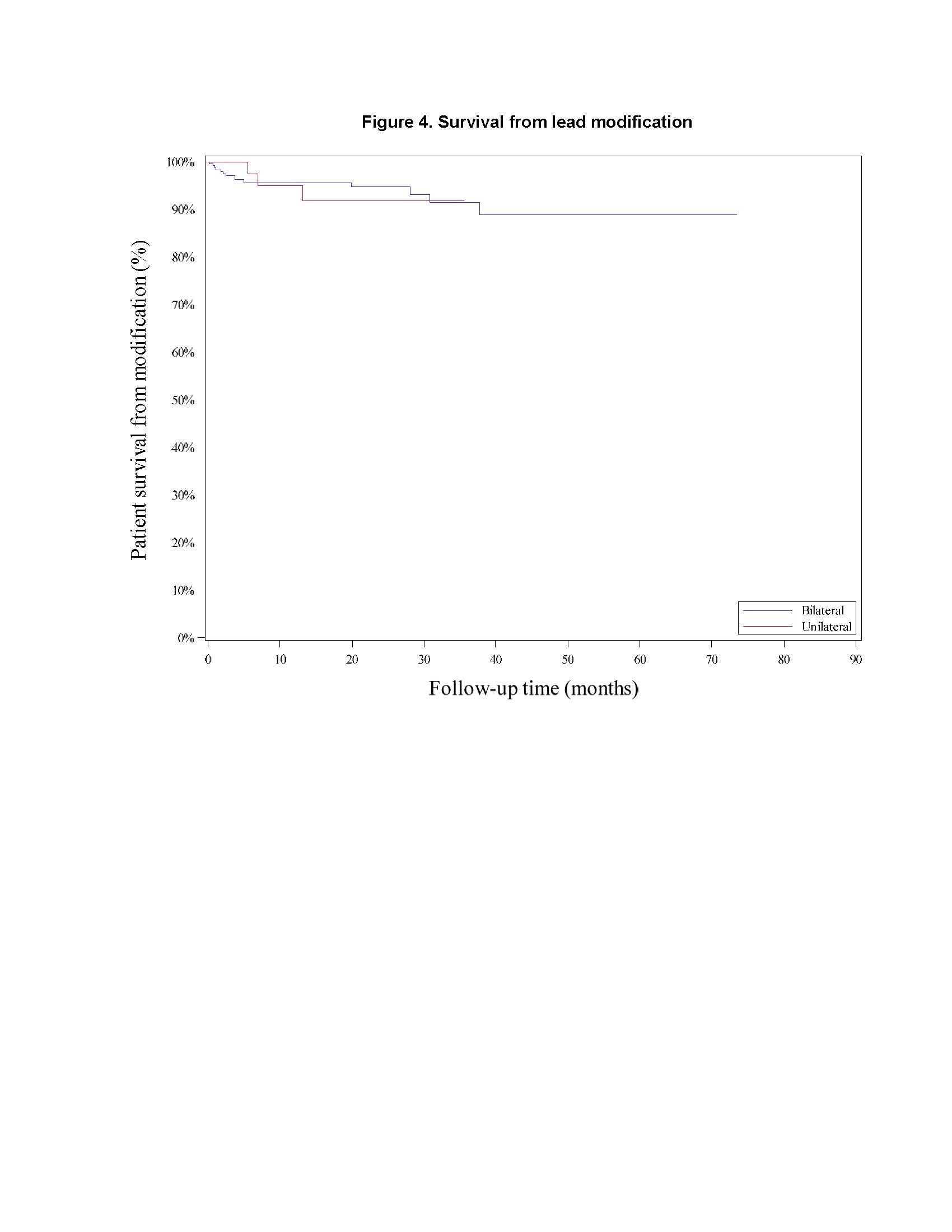
Results: There was no difference between cohorts in demographic/baseline characteristics [table1]. Average follow-up in years for combined Uni 2.2±1.4, 163 patient-years; combined Blt 2.0 ± 1.4, 646 patient years. Event rates per 100 patient years were similar between Uni and Blt cohorts for adverse events (AE), serious AE (SAE), Device Events (DE), Product Performance Related (PPR) DE, DE-lead related, PPR DE –lead related and Procedure Related AE. AE for rep in Blt cohort increased [table2] with trend toward more AE in rep Blt cohort after 12 months [figure1]. There were no dysarthria events in Uni, 4 (1.5%) Blt initial, 1(1.7%) Blt rep; balance disorder without events for Uni, 1 (0.4%) Blt initial. SAE by system organ class (SOC) by initial/ rep device status for Uni and Blt cohorts [table3] and time to first SAE [figure2] were similar. There were more DE in the rep Blt cohort [table 4], trend toward more AE in rep Blt cohort after 12 months [figure 3]. No significant difference between number of lead modifications between Uni and Blt cohorts [table5] with similar survival curves from lead modification [figure4].
Conclusions: Prospective analysis of patients in the PSR demonstrated that the overall AE and SAE were uncommon in both cohorts; with similar safety profiles demonstrated between Uni and Blt DBS implant patients with ET. This is in contrast to the reported literature in which dysarthria and balance /gait disorder events were more frequent in Blt compared to Uni DBS implanted ET patients.
References:
- Flora ED, Perera CL, Cameron AL, et al. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 2010; 25:1550–9.
2. Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, Fekete R, Jancovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond.
JNNP 2014; 85: 567-572.
To cite this abstract in AMA style:
M. Schiess, S. Falowski, G. Plotkin, S. Palfi, T. Witt, E. Cuny, T. Theys, K. Martinez, R. Plunkett, Y. Temel, G. Johnson, T. Weaver, J. Krauss, P. Konrad. Safety of Unilateral and Bilateral DBS implants in ET: Preliminary results from Product Surveillance Registry [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/safety-of-unilateral-and-bilateral-dbs-implants-in-et-preliminary-results-from-product-surveillance-registry/. Accessed December 31, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-of-unilateral-and-bilateral-dbs-implants-in-et-preliminary-results-from-product-surveillance-registry/